Figure 7.
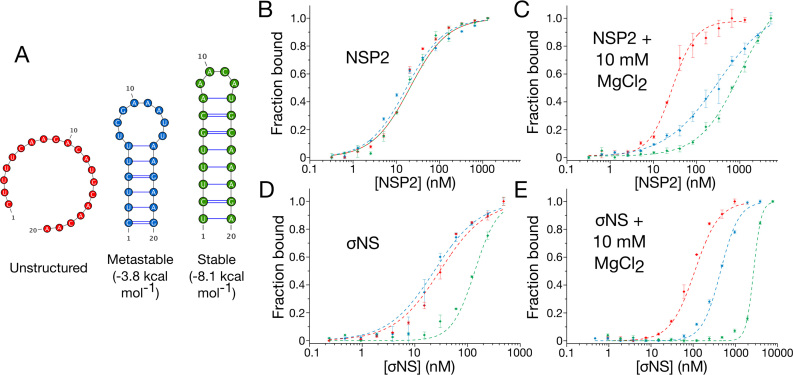
Stability of RNA structure determines preferential binding by NSP2 and σNS. (A) Fluorescently-labeled unstructured (red), metastable (blue) and stable (green) 20-mer RNAs used for fluorescence anisotropy binding assays. (B and D) NSP2 binds unstructured and stable RNAs with similar affinities. In contrast, stable secondary structure impedes σNS binding. (C and E) Mg2+-dependent stabilization of RNA structure impairs binding of ssRNAs by both NSP2 and σNS. Note the apparent affinity of both proteins for unstructured 20-mer remains largely unchanged upon addition of 10 mM MgCl2 Due to NSP2 aggregation at higher concentrations, protein titrations were only performed with [NSP2] up to 2 μM (C).