Abstract
Background
Many studies have confirmed that the size and location of necrotic lesions are major factors that affect the prevalence of collapse and prognosis in patients with osteonecrosis of the femoral head (ONFH). Although several classification systems categorize and quantify ONFH, there is no agreement on which one is most useful for the purpose.
Questions/purposes
We compared the Steinberg, modified Kerboul, and Japanese Investigation Committee (JIC) classifications of ONFH in terms of (1) the correlation among the three different classification systems. We further examined (2) the inter- and intraobserver reliability of the three classification systems and (3) the association of higher grades within each classification and the risk of subsequent collapse.
Methods
Between January 2000 and December 2014, we treated 101 hips in 74 patients for precollapse ONFH, diagnosed either on plain radiographs or MRI. Of those, one patient (1%) died, six patients (8%) were lost to followup, and two patients (3%) underwent osteotomy before 2 years, leaving 86 hips in 65 patients (88%) for analysis here. Three-dimensional spoiled gradient-echo sequence (3D-SPGR) MRI was performed for all hips, and the presence of ONFH was determined by finding the area surrounded by the outer margin of the low-signal-intensity band on 3D-SPGR MRI. Patients with ONFH were categorized using the Steinberg, modified Kerboul, and JIC classification systems, and correlations among these three classification systems were investigated. Inter- and intraobserver reliability was assessed by 10 orthopaedic surgeons using 40 sets of 3D-SPGR MR images. The reliability of each system was evaluated using the kappa coefficient. The cumulative survival rate with collapse and undergoing hip arthroplasty as the endpoints was evaluated for each of the three classification systems (mean followup, 9 years; range, 2–16 years), and the association of higher grades within each classification and the risk of subsequent collapse were also evaluated.
Results
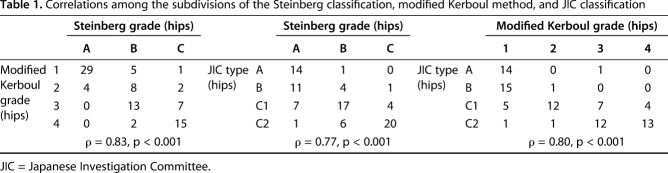
We found strong correlations between the Steinberg and modified Kerboul classifications (ρ = 0.83, p < 0.001), the Steinberg and JIC classifications (ρ = 0.77, p < 0.001), and the modified Kerboul and JIC classifications (ρ = 0.80, p < 0.001). Interobserver reliability in the JIC classification (0.72; range, 0.30–0.90) was higher than that in the Steinberg classification (0.56; range, 0.24–0.84; p < 0.001) and the modified Kerboul classification (0.57; range, 0.35–0.80; p < 0.001). The cumulative survival rate with collapse as the endpoint after a minimum of 2 years of followup in the Steinberg classification differed between Grades A (82%; 95% confidence interval [CI], 66%–97%) and B (43%; 95% CI, 21.9%–64.8%; p = 0.007), Grades A and C (20%; 95% CI, 4.3%–35.7%; p < 0.001), and Grades B and C (p = 0.029). Survival was lower for modified Kerboul Grade 4 hips (12%; 95% CI, 0%–27.1%) than for Steinberg Grade C hips (20%; 95% CI, 4.3%–35.7%) and JIC Type C2 hips (18%; 95% CI, 2.8%–34.0%). The JIC classification was best able to identify hips at low risk of collapse because no JIC Type A hips collapsed.
Conclusions
The JIC classification was more reliable and effective, at least for early-stage ONFH, than the Steinberg or modified Kerboul classifications. Further investigation might be useful to identify whether each classification system emphasizes specific risk factors for collapse.
Level of Evidence
Level III, diagnostic study.
Introduction
Osteonecrosis of the femoral head (ONFH) causes severe pain in affected patients, with collapse of the femoral head often leading to secondary osteoarthritis. The size and location of a necrotic lesion are important in determining the risk of collapse [2, 3, 8, 10, 11, 13, 14, 16, 19, 25-27, 29,30,31,32,33, 36, 38,39,40,41,42,43,44,45, 47], and several classification systems have been proposed to categorize and quantify ONFH on this basis [2, 3, 8, 10, 11, 16, 19, 23, 26, 33, 40, 42-44]. The Steinberg classification, initially published in 1984 [42], is based on lesion volume. Kerboul et al. proposed an angular method and initially classified hips based on radiographs [16], which has subsequently been modified to incorporate use of MRI [11, 19]. Finally, the Japanese Investigation Committee (JIC) classification is based on the location of the necrotic lesion [33, 44]. According to a systematic review of the natural history of untreated patients with asymptomatic ONFH, symptoms or collapse occurred in 394 (59%) of 664 hips, progression to collapse was indicated in 296 (49%) of 598 hips, and small and medially located necrotic lesions had a good prognosis [27].
However, there is no consensus regarding which of the classification systems in common use is most effective. In particular, there are few reports on the relationships among classifications based on volume, angle, or location [4, 13, 14, 25, 31, 39], and no report of which we are aware compares the three classification systems in the same patient group in terms of interobserver reliability or the association with subsequent collapse of the femoral head.
We therefore compared the Steinberg, modified Kerboul, and JIC classifications of ONFH in terms of (1) the correlation among the three classification systems. We further examined (2) the inter- and intraobserver reliability of the three classification systems and (3) the association of higher grades within each classification and the risk of subsequent collapse.
Patients and Methods
This retrospective study was approved by the local ethics commission. Between January 2000 and December 2014, we diagnosed ONFH before femoral head collapse in 101 hips in 74 patients. Fifteen hips in nine patients were excluded as a result of death (two hips in one patient), loss to followup (11 hips in six patients), or osteotomy within 2 years of diagnosis but before collapse (two patients in two hips). Therefore, 86 hips in 65 patients were available for followup at a minimum of 2 years (median, 9 years; range, 2-16 years) (Fig. 1). There were 24 men (30 hips) and 41 women (56 hips) with a mean age of 44.6 years (range, 14–81 years) and a mean body mass index of 21.8 kg/m2 (range, 16–35 kg/m2). ONFH was associated with steroid therapy in 51 patients (70 hips) and associated with alcohol abuse in 14 patients (16 hips). Three patients (four hips) received bisphosphonates after diagnosis of ONFH. All patients underwent clinical and radiographic examination with followup at 3- or 6-month intervals for the first 2 years and annually thereafter. MRI was performed in all patients using a 1.5-T General Electric superconducting unit (General Electric, Milwaukee, WI, USA). Three-dimensional spoiled gradient-echo sequence (3D-SPGR) was used in a coronal plane with a repetition time of 6 ms, echo time of 2 ms, a matrix size of 256 × 256, slice interval of 1 mm, and a field of view of 320 mm. All patients were imaged in a supine position, and the position of hips was unified in neutral abduction and adduction. The use of 3D-SPGR MRI is prevalent in musculoskeletal and organ image analyses, and it has several advantages over spin-echo imaging; these include high resolution, a high signal-to-noise ratio, thin imaging slices, and the ability to reconstitute images as needed [6, 18, 35]. The necrotic area was determined as the area surrounded by the outer margin of the low-signal-intensity band on 3D-SPGR MRI [31, 35].
Fig. 1.
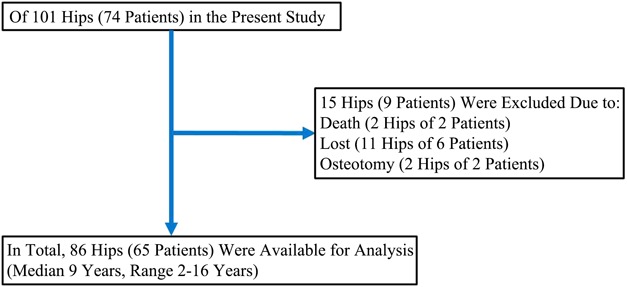
This is a flow diagram illustrating patient inclusion.
We investigated the relationships among the Steinberg classification, modified Kerboul method, and JIC classification by comparing the necrotic volume, combined necrotic angle, and necrotic lesion location. The necrotic volume was measured according to the Steinberg classification (Grade A, < 15%; Grade B, 15%–30%; Grade C, > 30%) using 3D-SPGR MRI (Fig. 2) [40]. The whole necrotic volume was calculated by taking a sum of the necrotic area of each slice and multiplying it by the interslice gaps using an open-source image processing program (Image J version 1.48; National Institutes of Health, Bethesda, MD, USA). We quantified the percent of necrotic volume by dividing the calculated necrotic volume by the whole femoral head volume, and we then categorized the Steinberg grade. We calculated the combined necrotic angle based on the modified Kerboul method and divided it into four categories: Grade 1 (< 200°), Grade 2 (200°–249°), Grade 3 (250°–299°), and Grade 4 (≥ 300°) (Fig. 3) [11]. Three-dimensional-SPGR MRI Digital Imaging and Communication in Medicine data were transferred to a personal computer and reconstructed from the coronal plane to the sagittal plane using computer software (Virtual Place version 1.163; AZE, Tokyo, Japan). We calculated the sum of the arc in the midcoronal and midsagittal images and then categorized the modified Kerboul grade. We categorized the location of the necrotic lesion at the weightbearing portion using the JIC classification, which is divided into four types (A, B, C1, and C2) (Fig. 4) [44]. Morphologic parameters for investigating the relationships among subdivisions in the three classification systems were also measured according to the following descriptions: (1) percentage of the necrotic area in the midcoronal image (necrotic area/whole femoral head area in the midcoronal image); (2) percentage of the necrotic area in the midsagittal image (necrotic area/whole femoral head area in the midsagittal image); and (3) the percentage of the largest necrotic area in the coronal image (the largest necrotic area/whole femoral head area in the midcoronal image) (Fig. 5). We evaluated correlations among the measured morphologic parameters.
Fig. 2.
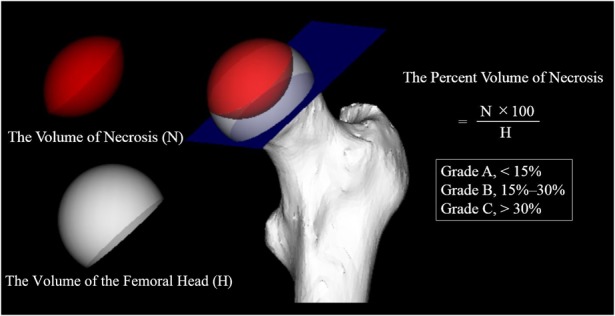
This is an illustration of the Steinberg grade.
Fig. 3 A-B.
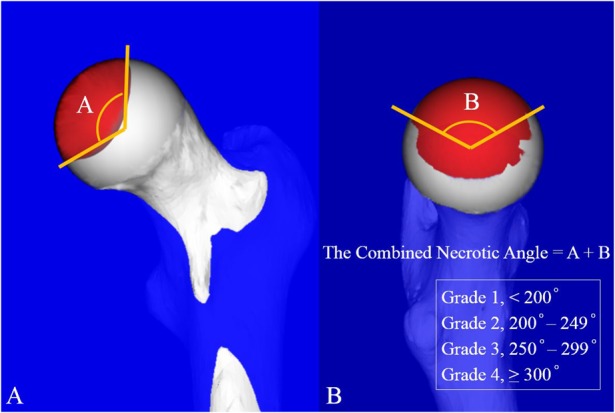
This is an illustration of the modified Kerboul grade. (A) The necrotic angle is in the midcoronal image, and (B) the necrotic angle is in the midsagittal image.
Fig. 4.
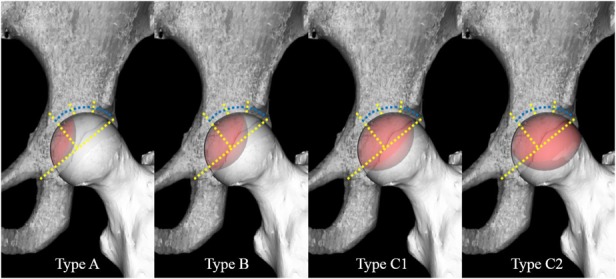
This is an illustration of JIC type. Type A lesions occupy the medial one-third or less of the weightbearing portion, Type B lesions occupy the medial two-thirds or less, Type C1 and Type C2 lesions both occupy more than the medial two-thirds; however, whereas Type C2 lesions extend laterally to the acetabular edge and Type C1 lesions do not. The weightbearing portion (blue dotted lines) is defined as the area lateral to the midvertical line of the line through the acetabular edge and teardrop bottom (yellow dotted lines).
Fig. 5.
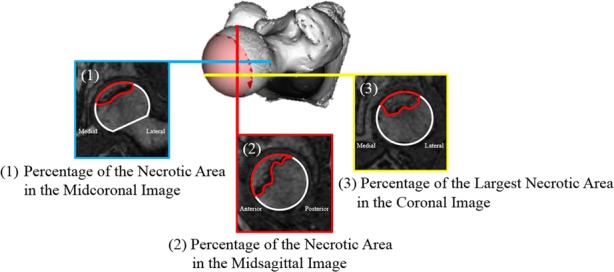
This is an illustration of the morphologic parameters that were measured in this study.
We followed the methods of Donner and Eliasziw [7] to evaluate inter- and intraobserver reliability. We chose a sample size of 40 sets of MR images to validate the evaluation. Inter- and intraobserver reliability was assessed by 10 orthopaedic surgeons with at least a 4-week interval between trials. Of the 10 observers, three were selected from other centers. Several studies have supported the validity of the number of necessary cases and observers [5, 11, 17, 19, 22, 28, 34, 37, 43]. Steinberg grades, modified Kerboul grades, and JIC types were independently categorized by each observer. We assessed the inter- and intraobserver reliability of the three classification systems using the kappa coefficient, which served as a standard for the strength of agreement. Kappa values were considered poor (< 0), slight (0–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect (0.81–1.00) [21].
We evaluated two types of cumulative survival rates: one with collapse as the endpoint and the other with undergoing hip arthroplasty as the endpoint. The cumulative survival rates were assessed for each of the three classification systems, and the reliability of categorizations in the three classification systems with the risk of subsequent collapse was estimated. The occurrence of collapse was investigated using AP and lateral radiographs evaluated by two orthopaedic surgeons. During this study, 39 of 86 hips (45%) collapsed, and the mean time between MRI examination and collapse was 24 months (range, 1–96 months). Of the 39 hips that collapsed, 28 (72%) hips collapsed within the initial 24 months. Meanwhile, hip arthroplasty was performed for 24 of 86 hips (28%; mean, 32 months; range, 3–97 months). All hip arthroplasties were performed on hips that had previously collapsed. Hip arthroplasty was indicated for patients who experienced unbearable, constant pain associated with collapse that limited their physical activity and who expressed a strong desire to undergo hip arthroplasty to reduce pain and improve activity.
Correlations in the subdivisions among the three classification systems were compared using Spearman’s rank correlation coefficient. Correlations of the morphologic parameters were compared using Spearman’s rank correlation coefficient. The classification systems and the measured morphologic parameters were set as independent variables with the remaining parameters set as dependent variables for evaluating these correlations. Kappa values of interobserver reliability among the three classification systems were compared using the Steel-Dwass test. Two types of cumulative survival rates were measured using the Kaplan-Meier method. The log-rank test was used to compare survival rates among the subdivisions in each classification system. Power analysis suggested that 82 hips would be required to achieve an adequate power of 0.8 for detecting minimum differences in the prediction of the prevalence of collapse. Results with p values of < 0.05 were considered to indicate differences or correlations. All statistical analyses were performed using SPSS software (version 23; IBM Japan, Tokyo, Japan).
Results
We found strong correlations among the three different classifications. Our comparisons included subdivisions of the Steinberg grade and modified Kerboul grade (ρ = 0.83, p < 0.001), the Steinberg grade and JIC type (ρ = 0.77, p < 0.001), and the modified Kerboul grade and JIC type (ρ = 0.80, p < 0.001) (Table 1). This was also the case when we compared necrotic area in the midcoronal image and that in the midsagittal image (ρ = 0.78, p < 0.001), necrotic area in the midcoronal image and largest necrotic area in the coronal image (ρ = 0.78, p < 0.001), and necrotic area in the midsagittal image and largest necrotic area in the coronal image (ρ = 0.84, p < 0.001).
Table 1.
Correlations among the subdivisions of the Steinberg classification, modified Kerboul method, and JIC classification
Inter- and intraobserver reliability was best for the JIC classification. Interobserver reliability was moderate for the Steinberg classification (mean, 0.56; range, 0.24–0.84) and modified Kerboul method (mean, 0.57; range, 0.35–0.80) and substantial for the JIC classification (mean, 0.72; range, 0.30–0.90), whereas intraobserver reliability was substantial for the Steinberg classification (mean, 0.78; range, 0.66–0.90) and modified Kerboul method (mean, 0.73; range, 0.64–0.84) and nearly perfect for the JIC classification (mean, 0.88; range, 0.83–0.97). The kappa values of interobserver reliability for the JIC classification (mean, 0.72; range, 0.30–0.90) were higher than those for the Steinberg classification (mean, 0.56; range, 0.24–0.84; p < 0.001) and modified Kerboul method (mean, 0.57; range, 0.35–0.80; p < 0.001).
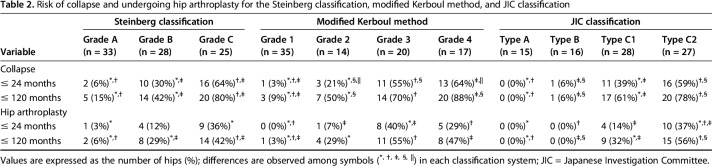
All classification systems had equivalent ability to identify a risk of collapse or use of THA in their higher grades. However, the JIC classification was the only system in which no hip classified as a more favorable grade collapsed or went on to a THA (Table 2). As the grades and types of the three classification systems increased in severity, the risk of collapse and hip arthroplasty increased. For the Steinberg classification, the cumulative survival rate with collapse as the endpoint after a minimum of 2 years of followup differed between Grades A (82%; 95% confidence interval [CI], 66.0%–97.0%) and B (43%; 95% CI, 21.9%–64.8%; p = 0.007), Grades A and C (20%; 95% CI, 4.3%–35.7%; p < 0.001), and Grades B and C (p = 0.029). The cumulative survival rate for undergoing hip arthroplasty as the endpoint was also different between Grades A (93%; 95% CI, 82.9%–100%) and B (65%; 95% CI, 44.4%–85.5%; p = 0.017), Grades A and C (41%; 95% CI, 20.3%–61.2%; p < 0.001), and Grades B and C (p = 0.046) (Fig. 6). The cumulative survival rate with collapse as the endpoint was lower for modified Kerboul Grade 4 hips (12%; 95% CI, 0%–27.1%) than for Steinberg Grade C hips (20%; 95% CI, 4.3%–35.7%) and JIC Type C2 hips (18%; 95% CI, 2.8%–34.0%) (Fig. 7). The JIC classification was best able to identify hips at a low risk of collapse because no JIC Type A hips collapsed, thereby producing cumulative survival rates of 100% for both endpoints in the JIC Type A hips (Fig. 8).
Table 2.
Risk of collapse and undergoing hip arthroplasty for the Steinberg classification, modified Kerboul method, and JIC classification
Fig. 6 A-B.
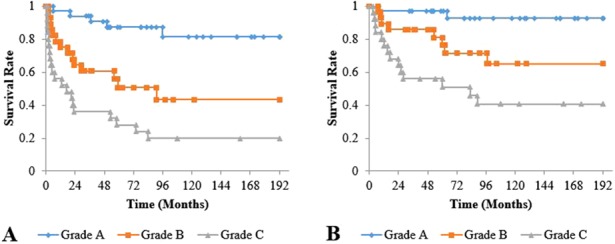
Graph (A) shows the cumulative survival rate with collapse as the endpoint and graph (B) shows those undergoing hip arthroplasty as the endpoint for Steinberg classification. (A) Survival rates at 10 years were 82% (95% CI, 66.0%–97.0%) for Grade A, 43% (95% CI, 21.9%–64.8%) for Grade B, and 20% (95% CI, 4.3%–35.7%) for Grade C; and differences were observed between Grades A and B (p = 0.007), Grades A and C (p < 0.001), and Grades B and C (p = 0.029). (B) Survival rates at 10 years were 93% (95% CI, 82.9%–100%) for Grade A, 65% (95% CI, 44.4%–85.5%) for Grade B, and 41% (95% CI, 20.3%–61.2%) for Grade C; and differences were observed between Grades A and B (p = 0.017), Grades A and C (p < 0.001), and Grades B and C (p = 0.046).
Fig. 7 A-B.
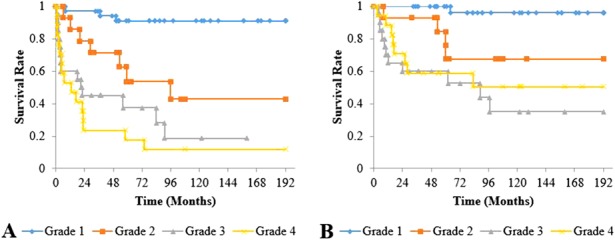
Graph (A) shows the cumulative survival rate with collapse as the endpoint, and graph (B) shows those undergoing hip arthroplasty as the endpoint for the modified Kerboul method. (A) Survival rates at 10 years were 91% (95% CI, 81.0%–100%) for Grade 1, 43% (95% CI, 13.7%–72.0%) for Grade 2, 19% (95% CI, 0%–40.3%) for Grade 3, and 12% (95% CI, 0%–27.1%) for Grade 4; and differences were observed between Grades 1 and 2 (p = 0.001), Grades 1 and 3 (p < 0.001), Grades 1 and 4 (p < 0.001), and Grades 2 and 4 (p = 0.008). (B) Survival rates at 10 years were 96% (95% CI, 88.8%–100%) for Grade 1, 68% (95% CI, 41.2%–93.9%) for Grade 2, 35% (95% CI, 9.8%–60.2%) for Grade 3, and 50% (95% CI, 25.2%–75.6%) for Grade 4; and differences were observed between Grades 1 and 2 (p = 0.006), Grades 1 and 3 (p < 0.001), and Grades 1 and 4 (p < 0.001).
Fig. 8 A-B.
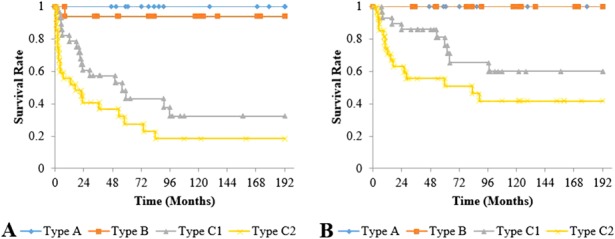
Graph (A) shows the cumulative survival rate with collapse as the endpoint, and graph (B) shows those undergoing hip arthroplasty as the endpoint for JIC classification. (A) Survival rates at 10 years were 100% for Type A, 94% (95% CI, 81.9%–100%) for Type B, 32% (95% CI, 12.8%–51.9%) for Type C1, and 18% (95% CI, 2.8%–34.0%) for Type C2; and differences were observed between Types A and C1 (p < 0.001), Types A and C2 (p < 0.001), Types B and C1 (p = 0.001), and Types B and C2 (p < 0.001). (B) Survival rates at 10 years were 100% for Types A and B, 60% (95% CI, 38.9%–81.0%) for Type C1, and 42% (95% CI, 22.1%–61.2%) for Type C2; and differences were observed between Types A and C1 (p = 0.016), Types A and C2 (p < 0.001), Types B and C1 (p = 0.012), and Types B and C2 (p < 0.001).
Discussion
Although several classification systems categorize and quantify ONFH, there is no consensus regarding which of the classification systems in common use is most effective. In particular, no report of which we are aware compares the three classification systems in the same patient group in terms of inter- and intraobserver reliability or the association with subsequent collapse of the femoral head. We found that reliability was best for the JIC classification and that the JIC classification was the only system in which no hip classified as a more favorable grade collapsed or went on to a THA. The JIC classification was more reliable and effective at least for early-stage ONFH than Steinberg or Kerboul classifications.
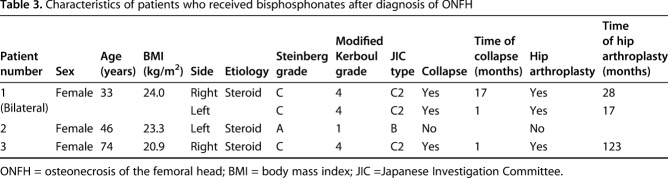
This study had several limitations. First, this study included patients with ONFH associated with steroid therapy and alcohol abuse. The proportion of steroid- and alcohol-associated ONFH is particular to studies in Asia [9, 15, 46] and might not be necessarily typical of studies elsewhere. In addition, patients with sickle cell disease, whose prognosis is considered poor, are less frequent in Japan [9, 12, 24]. However, the proportion of patients who sustained femoral head collapse in this study (45%) was similar to those reported in a systematic review (49%) [27]. We believe that our results probably generalize to other populations. Second, this study did not evaluate whether patients with ONFH were initially symptomatic or asymptomatic. However, it is generally known that when the femoral head collapses, patients with ONFH become symptomatic [20]. Furthermore, none of the patients without collapse underwent hip arthroplasty in this study. Third, three of 65 patients in this study received bisphosphonates after a diagnosis of ONFH (Table 3). However, the effect of bisphosphonates in patients with ONFH is controversial. A previous study demonstrated that bisphosphonates improved function and survival in patients with ONFH [1], whereas a multicenter, randomized controlled trial showed that bisphosphonates had no effect on collapse prevention, disease progression, or hip arthroplasty compared with a placebo [4]. Fourth, the individuals evaluating MRIs in our study were not of equal experience or degree of specialization. However, there was no difference in the inter- or intraobserver reliability between subspecialists and others. Finally, this study did not evaluate the degree or the extent of collapse. Nishii et al. [30] reported that even if collapse occurs, subsequent cessation of collapse can be expected in certain patients. They observed cessation of collapse in patients with small necrotic lesions occupying less than the medial two-thirds of the weightbearing portion, and they found that patients with < 2 mm of collapse could possibly become asymptomatic. Further investigation is necessary to identify the factor(s) responsible for differences between patients with progression and cessation of collapse.
Table 3.
Characteristics of patients who received bisphosphonates after diagnosis of ONFH
This study is the first report to compare the three classification systems in the same patient group in terms of inter- and intraobserver reliability and the association with subsequent collapse of the femoral head. We found strong correlations among the subdivisions of the three different classifications. Steinberg et al. [39] have proposed that volumetric measurement is more reliable and more accurate than angular measurement; however, they have reported the correlation between the volumetric and angular methods without evaluating the reliability of these methods for prognosis. Koo and Kim [19] and Ha et al. [11] have concluded that the angular methods are more acceptable for clinical use than the Steinberg classification based on the necrotic volume because the former methods are simple and easy to use. They have also indicated that the angular methods are more accurate in predicting the occurrence of collapse than methods of measurement in a single plane. However, they have not compared the angular systems directly with volumetric and locational classification systems.
No report of which we are aware compares the three classification systems in the same patient group in terms of inter- and intraobserver reliability. This study demonstrated that reliability was best for the JIC classification. A reliability coefficient of 81.9% for the Steinberg classification was reported for three independent observers [43]; however, the volume measurement of ONFH is time-consuming if special software cannot be used [31, 40]. Although a previous study demonstrated that the coefficient of variation of estimation in the angular methods was 4.3% for 10 independent orthopaedic surgeons [11], the other study reported that the diffuse and patchy case of necrotic lesions was difficult to calculate necrotic angle [5]. Previous work has shown that the JIC classification is reproducible with inter- and intraobserver reliability of 82% and 80%, respectively, for MRI on a 1.5-T unit. Moreover, no difference was observed in the inter- and intraobserver reliability between 0.5-T and 1.5-T units [28]. The JIC classification is the simplest method of the three classification systems because lesions are categorized with respect to the midcoronal image alone. Therefore, the JIC classification appears to be the most reliable.
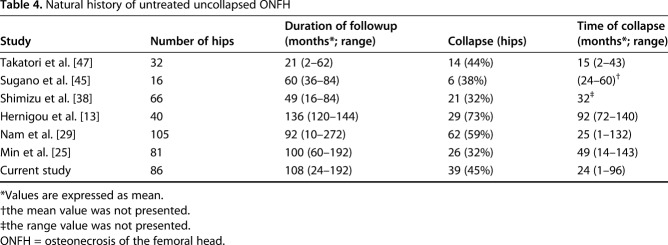
Several studies regarding the JIC classification have indicated that the location of the necrotic lesion relative to the acetabular weightbearing portion is an important factor for the association with subsequent collapse [13, 14, 29,30,31,32,33, 36, 38, 45, 47]. Although the size and location of necrotic lesions are considered independently related factors of collapse, quantitative analysis of necrotic lesion morphology revealed that the necrotic volume is correlated with the risk of collapse and that the location of necrotic lesions is an important prognostic factor of collapse even if the necrotic volume is small [13, 31]. According to the natural history in patients with ONFH, but without collapse, using an MRI-based evaluation, most collapses were observed within the initial few years [29, 38, 45, 47]; however, other studies have found that some collapses occurred after long-term followup (Table 4) [13, 25]. We found that severe grades and types of hips such as Steinberg Grade C, modified Kerboul Grade 4, and JIC Type C2 hips had similar collapse appearance times. However, some Steinberg Grade A and modified Kerboul Grade 1 hips collapsed during midterm followup, resulting in hip arthroplasty. The finding that no JIC Type A hips collapsed can help surgeons evaluate the prognosis and select treatment for ONFH.
Table 4.
Natural history of untreated uncollapsed ONFH
We conclude that the JIC classification was more reliable and effective at least in early-stage ONFH than the Steinberg or modified Kerboul classifications. Further investigation might be useful to identify whether each classification system emphasizes specific risk factors for collapse.
Acknowledgments
We thank Drs Ichiro Nakahara, Hirohito Abe, Keisuke Uemura, Ryota Nakaya, and Kazunori Tamura for their helpful support.
Footnotes
The principal investigator (NS) of the Japanese Investigation Committee under the auspices of the Ministry of Health Labour and Welfare received a research grant from the Ministry of Health and Welfare of Japan (Research on Measures for Intractable Diseases).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agarwala S, Shah SB. Ten-year follow-up of avascular necrosis of femoral head treated with alendronate for 3 years. J Arthroplasty. 2011;26:1128–1134. [DOI] [PubMed] [Google Scholar]
- 2.ARCO (Association Research Circulation Osseous): Committee on terminology and classification. ARCO News. 1992;4:41–46. [Google Scholar]
- 3.Arlet J, Ficat P, Sebbag D. The use of measurement of intramedullary pressure in the greater trochanter in man, particularly in the diagnosis of osteonecrosis of the femoral head. Rev Rhum Mal Osteoartic. 1968;35:250–256. [PubMed] [Google Scholar]
- 4.Chen CH, Chang JK, Lai KA, Hou SM, Chang CH, Wang GJ. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: a two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–1578. [DOI] [PubMed] [Google Scholar]
- 5.Cherian SF, Laorr A, Saleh KJ, Kuskowski MA, Bailey RF, Cheng EY. Quantifying the extent of femoral head involvement in osteonecrosis. J Bone Joint Surg Am. 2003;85:309–315. [DOI] [PubMed] [Google Scholar]
- 6.Disler DG, Peters TL, Muscoreil SJ, Ratner LM, Wagle WA, Cousins JP, Rifkin MD. Fat-suppressed spoiled GRASS imaging of knee hyaline cartilage: technique optimization and comparison with conventional MR imaging. AJR Am J Roentgenol. 1994;163:887–892. [DOI] [PubMed] [Google Scholar]
- 7.Donner A, Eliasziw M. Sample size requirements for reliability studies. Stat Med. 1987;6:441–448. [DOI] [PubMed] [Google Scholar]
- 8.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 2010;468:2715–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardeniers JWM. Report of the committee of staging and nomenclature. ARCO News Letter. 1993;5:79–82. [Google Scholar]
- 11.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88:35–40. [DOI] [PubMed] [Google Scholar]
- 12.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–2572. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P, Poignard A, Nogier A, Manicom O. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86:2589–2593. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Matsuno T, Omizu N, Aoki Y, Minami A. Mid-term prognosis of non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 2003;85:796–801. [PubMed] [Google Scholar]
- 15.Kang JS, Moon KH, Kwon DG, Shin BK, Woo MS. The natural history of asymptomatic osteonecrosis of the femoral head. Int Orthop. 2013;37:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerboul M, Thomine J, Postel M, Merle d’Aubigné R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291–296. [PubMed] [Google Scholar]
- 17.Kim YM, Ahn JH, Kang HS, Kim HJ. Estimation of the extent of osteonecrosis of the femoral head using MRI. J Bone Joint Surg Br. 1998;80:954–958. [DOI] [PubMed] [Google Scholar]
- 18.Kishida Y, Nishii T, Sugano N, Nakanishi K, Sakai T, Miki H, Ochi T, Yoshikawa H. Measurement of lesion area and volume by three-dimensional spoiled gradient-echo MR imaging in osteonecrosis of the femoral head. J Orthop Res. 2003;21:850–858. [DOI] [PubMed] [Google Scholar]
- 19.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77:875–880. [PubMed] [Google Scholar]
- 20.Kubo T, Yamazoe S, Sugano N, Fujioka M, Naruse S, Yoshimura N, Oka T, Hirasawa Y. Initial MRI findings of non-traumatic osteonecrosis of the femoral head in renal allograft recipients. Magn Reson Imaging. 1997;15:1017–1023. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 22.Loewen S, Philp J. Recasts in the adult L2 classroom: characteristics, explicitness, and effectiveness. Mod Lang J. 2006;90:536–556. [Google Scholar]
- 23.Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting, J Bone Joint Surg Am. 1973;55:1351–1366. [PubMed] [Google Scholar]
- 24.Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, Bellevue R, Koshy M, Moohr JW, Smith J. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–1481. [DOI] [PubMed] [Google Scholar]
- 25.Min BW, Song KS, Cho CH, Lee SM, Lee KJ. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, Steinberg ME. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88:16–26. [DOI] [PubMed] [Google Scholar]
- 27.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92:2165–2170. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura J, Kishida S, Harada Y, Iida S, Oinuma K, Yamamoto S, Nakajima T, Takazawa M, Shigemura T, Ohtori S, Sato Y, Takahashi K. Inter-observer and intra-observer reliabilities of the Japanese Ministry of Health, Labor and Welfare type classification system for osteonecrosis of the femoral head. Mod Rheumatol. 2011;21: 488–494. [DOI] [PubMed] [Google Scholar]
- 29.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90:477–484. [DOI] [PubMed] [Google Scholar]
- 30.Nishii T, Sugano N, Ohzono K, Sakai T, Haraguchi K, Yoshikawa H. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2002;400:149–157. [DOI] [PubMed] [Google Scholar]
- 31.Nishii T, Sugano N, Ohzono K, Sakai T, Sato Y, Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J Orthop Res. 2002;20:130–136. [DOI] [PubMed] [Google Scholar]
- 32.Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73:68–72. [DOI] [PubMed] [Google Scholar]
- 33.Ono K. Diagnostic criteria, staging system, and roentgenographic classification of avascular necrosis of the femoral head (steroid induced, alcohol associated, or idiopathic nature). In: Ono K, ed. Annual Report of Japanese Investigation Committee for Intractable Diseases, Avascular Necrosis of the Femoral Head. Tokyo, Japan: Ministry of Health Welfare; 1987:331–336. [Google Scholar]
- 34.Plakseychuk AY, Shah M, Varitimidis SE, Rubash HE, Sotereanos D. Classification of osteonecrosis of the femoral head. Reliability, reproducibility, and prognostic value. Clin Orthop Relat Res. 2001;386:34–41. [DOI] [PubMed] [Google Scholar]
- 35.Sakai T, Sugano N, Nishii T, Haraguchi K, Ochi T, Ohzono K. MR findings of necrotic lesions and the extralesional area of osteonecrosis of the femoral head. Skeletal Radiol. 2000;29:133–141. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto M, Shimizu K, Iida S, Akita T, Moriya H, Nawata Y. Osteonecrosis of the femoral head: a prospective study with MRI. J Bone Joint Surg Br. 1997;79:213–219. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt SM, Kirchhoff C, Mayer W, Goebel M, Jansson V. Avascular necrosis of the femoral head: inter- and intraobserver variations of Ficat and ARCO classifications. Int Orthop. 2008;32:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu K, Moriya H, Akita T, Sakamoto M, Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Bone Joint Surg Am. 1994;76:215–223. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg DR, Steinberg ME, Garino JP, Dalinka M, Udupa JK. Determining lesion size in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88:27–34. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–271. [PubMed] [Google Scholar]
- 41.Steinberg ME, Brighton CT, Steinberg DR, Too SE, Hayken GD. Treatment of avascular necrosis of the femoral head by a combination of grafting, decompression and electrical stimulation. Clin Orthop Relat Res. 1984;186:137–153. [PubMed] [Google Scholar]
- 42.Steinberg ME, Hayken GD, Steinberg DR. A new method for evaluation and staging of avascular necrosis of the femoral head. In: Arlet J, Ficat RP, Hungerford DS, eds. Bone Circulation. Baltimore, MD, USA: Williams & Wilkins; 1984:398–403. [Google Scholar]
- 43.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 44.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci. 2002;7:601–605. [DOI] [PubMed] [Google Scholar]
- 45.Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994;305:190–199. [PubMed] [Google Scholar]
- 46.Takahashi S, Fukushima W, Yamamoto T, Iwamoto Y, Kubo T, Sugano N, Hirota Y. Temporal trends in characteristics of newly diagnosed nontraumatic osteonecrosis of the femoral head from 1997 to 2011: a hospital-based sentinel monitoring system in Japan. J Epidemiol. 2015;25:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takatori Y, Kokubo T, Ninomiya S, Nakamura S, Morimoto S, Kusaba I. Avascular necrosis of the femoral head. Natural history and magnetic resonance imaging. J Bone Joint Surg Br. 1993;75:217–221. [DOI] [PubMed] [Google Scholar]