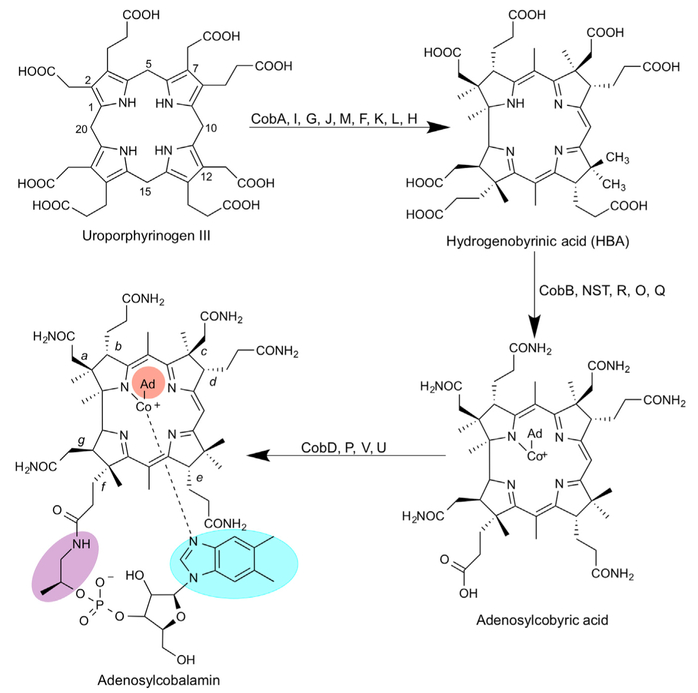
Figure 1. Biosynthesis of Adenosylcobalamin from Uroporphyrinogen III.
Initially, uroporphyrinogen III is acted upon by the enzymes in the sequence of CobA, I, G, J, M, F, K, L, and H to generate a cobalt-free corrinoid called HBA. Amidation of the a and c side chains followed by metal insertion, adenosylation, and the remaining amidations by CobB, NST, R, O, and Q gives rise to adenosylcobyric acid. The final stages of the biosynthesis see the attachment of an aminopropanol side chain and the alpha ribazole nucleotide, which contains the unusual base dimethylbenzimidazole. These reactions are mediated by the enzymes CobD, P, V, and U. The numbering of the corrin macrocyle (1–20) is shown as is the lettering associated with the side chains (a-g). Different variants of cobalamin are found through changes in the highlighted areas of the molecule. The upper ligand (orange) can be a methyl, cyano, or water group, the linking aminopropanol (purple) is an ethanolamine molecule in nor-cobalamin, and a range of different bases can replace the dimethylbenzimidazole (blue).