Abstract
Although connectivity can promote host species persistence in a metapopulation, dispersal may also enable disease transmission, an effect further complicated by the impact that parasite distribution may have on host–parasite population dynamics. We investigated the effects of connectivity and initial parasite distribution (clustered or dispersed) on microparasite–host dynamics in experimental metapopulations, using guppies and Gyrodactylus turnbulli. We created metapopulations of guppies divided into four subpopulations and introduced either a low level of parasites to all subpopulations (dispersed) or a high level of parasites to one subpopulation (clustered). Controlled migration among subpopulations occurred every 10 days. In additional trials, we introduced low or high levels of parasites to isolated populations. Parasites persisted longer in metapopulations than in isolated populations. Mortality was lowest in isolated populations with low-level introductions. The interaction of connectivity and initial parasite distribution influenced parasite abundance. With low-level introductions, connectivity helped the parasite persist longer but had little effect on the hosts. With high levels, connectivity also benefited the hosts, lowering parasite burdens. These findings have implications for disease management and species conservation.
Keywords: metapopulation ecology, asynchrony, parasite distribution, host–parasite dynamics, guppies, Gyrodactylus
1. Introduction
Infectious disease [1] and habitat fragmentation [2] both contribute to species decline and therefore have important implications for conservation. Although connectivity and migration among populations promote species persistence in metapopulations [3,4], dispersal made possible by connectivity may also act as an agent for disease transmission, either through continued introduction of susceptible hosts to infected populations or introduction of parasites to naive populations [5–7]. Therefore, connectivity may also negatively impact host survival, if pathogens are present within the system. Despite a rich theoretical literature on disease dynamics in metapopulations [5,8–10], few empirical studies test the theoretical predictions [11,12]. To better understand the impacts of host population connectivity on host–parasite dynamics, laboratory experiments that manipulate connectivity are necessary.
Across a metapopulation, asynchrony in population dynamics among patches may further prolong species persistence by reducing the risk of extinction overall [4,13]. For parasitic infections, particularly those that confer immunity, asynchrony in dynamics can be a major contributor to prolonging parasite persistence by allowing parasites to thrive in some patches despite the presence of resistant hosts in others [14]. Therefore, within a metapopulation context, the way in which parasites are initially distributed across host populations may impact parasite dynamics.
We set out to determine the impacts of connectivity on parasite–host dynamics, ultimately asking whether connectivity benefits hosts, parasites or both. To tailor this question to different initial parasite distributions, we additionally considered the effects of either clustered, high local abundance infection where parasites were introduced into a single host sub-population at high levels, or dispersed, low local abundance infection where parasites were introduced evenly to host sub-populations at low levels. To answer these questions, we used the guppy–Gyrodactylus turnbulli host–parasite system [15].
Gyrodactylus spp. are monogenean ectoparasites of fish that reproduce rapidly on the surface of the fish via polyembryony. Transmission occurs through direct skin-to-skin contact between live hosts [16]. They have no free-living stage, though detached Gyrodactylus sp. may survive for up to 12 h [15] and can potentially reattach to a host [17]. Gyrodactylids have been shown to cause severe disease and high mortality rates in aquaculture settings [18,19], and in the laboratory [20,21]. However, some hosts are able to clear their infection, after which they are refractory to reinfection until their immunity wanes [22–24]. Gyrodactylids persist in the wild at low levels periodically causing epidemics with fluctuations in the number of susceptible, infected and recovered hosts in the population [25]. They can be counted without destructive sampling of the host and their numbers can be tracked on individual hosts over time [15,26], making them convenient subjects for the study of host–parasite metapopulation dynamics.
Guppies (Poecilia reticulata) are a common freshwater fish and the specific host of Gyrodactylus turnbulli [25]. Although it has been shown that immigration of susceptible hosts into laboratory guppy populations is necessary for gyrodactylid persistence [15], and that in the field, downstream guppy dispersal is more likely for heavily infected guppies [27], the effects of host dispersal on disease dynamics at the metapopulation level have not been examined in this host–parasite system. Also, the impact of initial infection burden of Gyrodactylus spp. on parasite dynamics on individual guppies has been investigated [28], but not the impact of initial introduction levels or distribution on host–parasite dynamics within a metapopulation.
The goal for this experiment was to test the effects of connectivity and the initial parasite distribution throughout connected metapopulations on parasite persistence, host–parasite population dynamics, and host mortality. We predicted that parasites would persist longer in connected metapopulations than in isolated control populations, as movement through the metapopulations would provide the parasite with access to susceptible fish. We also predicted that focal introduction of parasites into only one subpopulation rather than simultaneous introduction into all the subpopulations would further prolong parasite persistence in the metapopulation by forcing asynchrony in local parasite dynamics among tanks. Finally, we hypothesized that parasite abundance would reach higher peaks in tanks into which more parasites had been initially introduced, resulting in higher host mortality, and that this would be more evident in isolated compared to connected populations because parasites would be unable to spread out and would thus be constrained to a local population.
2. Methods
(a). Experimental design
This experiment consisted of two types of metapopulations, each containing four tanks of eight fish with two distinct starting conditions: either two parasites introduced into each tank at the same time (low/dispersed parasites, connected) or eight parasites introduced into only one of the four tanks (high/clustered parasites, connected). Every 10 days, one fish was haphazardly selected from each tank in these connected metapopulations and moved to the next tank in a unidirectional loop (A → B → C → D → A). A diagram of the experimental design can be found in electronic supplementary material, figure S1. The 10-day interval was chosen to coincide with anticipated major epidemic parameters such as peak prevalence and abundance after 10 days, the end of the epidemic after 20 days [29], and the waning of most acquired immunity after 40 days [22]. To control for connectivity, we also included two types of isolated tanks which were not part of any metapopulation (no dispersal was applied), into which either two (low parasites, isolated) or eight (high parasites, isolated) parasites were introduced. The full experiment was replicated four times, in a total of five blocks of trials due to manpower constraints.
(b). Background
Guppies were purchased from a Montreal pet store and brought to aquariums in a McGill University laboratory maintained at 26 ± 1°C and a 12 L : 12 D cycle where they were bred for two generations. Upon receipt, fish had low levels of infection (less than 20 parasites on about 20% of the fish), and parasite transmission continued in our breeding stock. Gyrodactylus turnbulli were obtained from an infected pet store guppy and cultured in the laboratory by infecting one naive guppy with one parasite and routinely adding naive fish. This parasite culture has been maintained for several years and identified as G. turnbulli.
Only adult male guppies were used for the experiment. Fish (mean weight 0.125 g, standard deviation 0.043) were haphazardly selected from our breeding stock tank, assigned to groups of 8 and placed in 6 l tanks in an Aquaneering, Inc. (San Diego, CA, USA) flow-through system. As transmission rates are affected by host density [26], it is important to note that this density of fish is higher than wild populations [30], but lower than in commercial guppy populations [31], and similar to those used in laboratory epidemic experiments [29,32]. Prior to the experiment, fish were treated twice at a one-week interval with 25 g l−1 salt water for 15 min [33] to eliminate Gyrodactylus. They were then anaesthetized in 0.02% tricaine methanesulfonate (MS-222) buffered to a neutral pH with sodium bicarbonate (Sigma-Aldrich, Darmstadt, Germany) and scanned using a dissecting microscope with a cold light-source to confirm the absence of Gyrodactylus. Fish were maintained in their group tanks for eight weeks to ensure that all fish had overcome the refractory period to any potential prior Gyrodactylus infection [22,23,34]. Tanks were haphazardly assigned to one of the four experimental groups. Every day throughout the experiment, fish were fed a controlled amount of TetraMin Tropical Flakes (Tetra Werke, Melle Germany) mixed with water into a paste that was distributed through a glass precision syringe to each tank.
(c). Experimental protocol
One week before parasite introductions, anaesthetized fish were injected with visible implant elastomer dye (Northwest Marine Technologies, Shaw Island, WA, USA) for unique identification. On day 0 of the experiment, all fish were anaesthetized, weighed to 0.001 grams, and measured to 0.1 mm. Fish were infected by removing a scale from a heavily infected donor fish and placing it on the recipient fish until the parasites had transferred to the new host [29,35]. Two parasites per fish was chosen as the infection dose to increase the probability of parasite establishment in the tank and still allow detection of changes in the initial population growth rate over the first several days [28].
Each fish was then anaesthetized and scanned for parasites every other day. Movement in the connected metapopulations occurred after parasites had been counted, according to experimental design. Dead fish were not replaced, to avoid altering dynamics by introducing naive fish. If all fish in a tank died, the tank was left as an unoccupied ‘patch’ that would be ‘recolonized’ by a fish and potentially parasites at the next 10-day interval. Metapopulation trials lasted 120 days (three full cycles of dispersal) or until no parasites were found in any connected tanks for two consecutive counts. Isolated tanks were monitored until no parasites were found for two consecutive counts.
(d). Statistical analysis
Analyses were conducted at the system level; thus our experimental units were metapopulations and isolated tanks. For each metapopulation, the aggregation of parasites among the four connected tanks was calculated using the variance to mean ratio of the total parasites per tank on each day. For each metapopulation and isolated tank, parasite prevalence (number of infected hosts per total number of hosts) was also calculated for each day. The total duration of parasite persistence in the metapopulation and isolated tank, host mortality (the proportion of fish that died in the metapopulation or isolated tank), the maximum total number of parasites in the metapopulation or isolated tank, the daily and peak mean abundance (total number of parasites per fish in the metapopulation or isolated tank), and peak prevalence were recorded over the course of the experiment.
All analyses were performed in R v. 3.2.2 [36]. To assess the effect of connectivity (connected versus isolated), parasite introduction (low/dispersed versus high/clustered) and their interaction on our system-level response variables (persistence, mortality, peak total parasites and peak mean abundance) we used generalized linear mixed-effect models (GLMM) (function glmer, package lme4), with the block in which a trial was run treated as a random effect, the interaction of connectivity and parasite introduction as a fixed effect, and with different error distributions depending on the nature of the data. We simplified full models first by removing the interaction term if not significant, then each term (connectivity or parasite introduction) individually, comparing AICs with the full model at each step to find the model with the lowest AIC. Absolute goodness of fit of the minimal models was assessed as R2, by calculating the correlation between the observed and fitted values, squared.
Different response variables had different error distributions given the aggregated nature of parasite load among hosts, and the binomial nature of mortality, therefore different error distributions were applied to our models for each response variable. For overall parasite persistence in a system, we used a GLMM with a Poisson error distribution. For mortality, we used a GLMM with a binomial error distribution. For the peak total number of parasites, we used a GLMM with a negative binomial error distribution. For peak mean abundance, we used a GLMM with a negative binomial error distribution.
Reported results are means associated with standard error. Here α was set at 0.05. We report the R2-value of each minimal model and the p-value of significant variables.
3. Results
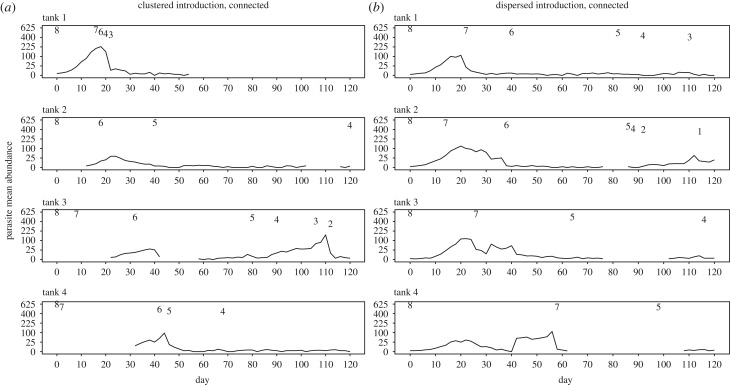
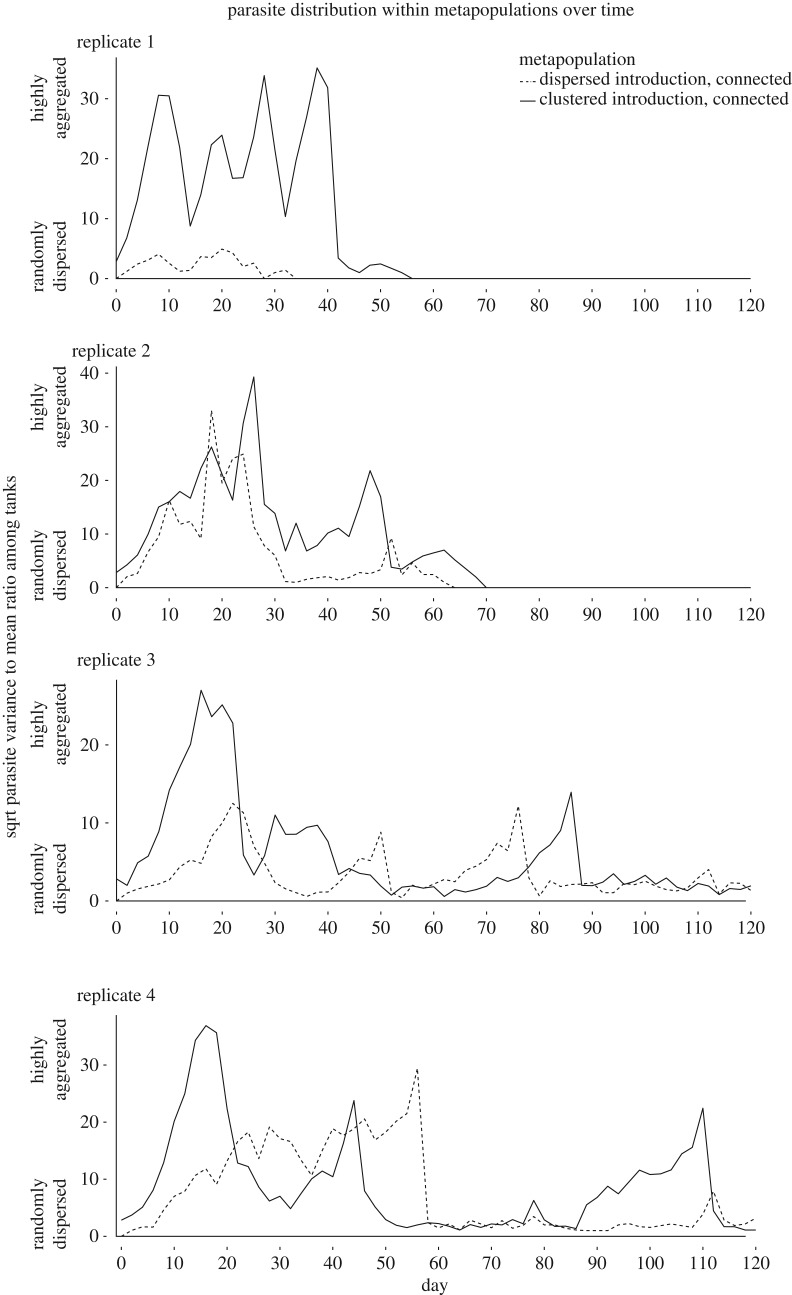
Minimal models and their outputs are summarized in electronic supplementary materials (S3). Within each replicate, fish size did not significantly differ among treatments (p > 0.05 for all comparisons of weight and standard length among treatments). In all tanks, parasite populations increased and spread throughout the host population, reaching at least one distinct population peak. In all our initially clustered metapopulations, the dispersal of an infected fish to a naive tank resulted in parasite populations establishing in the new tank, generating asynchrony among tanks during the first 30 days (figure 1). All eight isolated tanks (both high and low introductions) reached 100% prevalence, two of the four metapopulations with a dispersed parasite introduction reached 100% prevalence (with the other two reaching a maximum of 37.5 and 96.8%), and none of the metapopulations with a clustered introduction reached 100% prevalence (76.9, 84.3, 76.1 and 86.3%). For both clustered and dispersed metapopulations, the variance to mean ratio of parasites among tanks reached high levels and fluctuated over time in a similar manner, despite different initial values (figure 2).
Figure 1.
Mean parasite abundance over time (square-root scaled for graphing purposes) from one representative replicate of the (a) high parasite clustered metapopulation and (b) low parasite dispersed metapopulation. Fish numbers are recorded when they change across the top of each graph. Note that single instances of zero-values may not represent true zeros for the parasite population, as detection is not perfect and fish may be cryptically infected from which parasite populations may resurge. Zero-values for two or more consecutive counting days are assumed to be true zeros.
Figure 2.
Variance-to-mean ratio of total parasite numbers among tanks in each replicate set of clustered (solid) and dispersed (dashed) metapopulations, square-root transformed for graphing purposes.
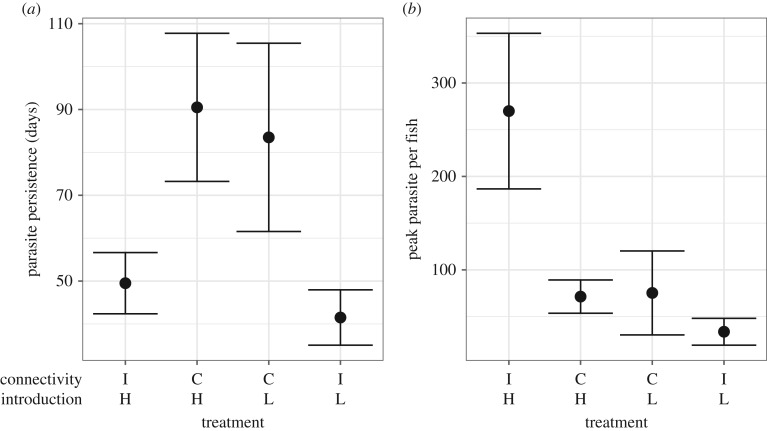
No interactions of connectivity and parasite introduction were detected for parasite persistence or host mortality. Connectivity influenced parasite persistence (p < 0.001, model R2 = 0.9), with parasite populations lasting an average of 87 ± 13 days in connected metapopulations, compared with 45 ± 5 days in isolated tanks (figure 3). Parasite introduction (p = 0.001) and connectivity (p = 0.009) influenced host mortality (model R2 = 0.84) with high initial levels leading to greater mortality (61 ± 8%), than low ones (34.8 ± 11%), and isolated tanks having lower mortality (43.7 ± 11%) than connected metapopulations (51.9 ± 10%).
Figure 3.
Effects of connectivity (‘C’ = connected, ‘I’ = isolated) and parasite introduction (‘H’ = high/clustered, ‘L’ = low/dispersed) on (a) parasite persistence and (b) mean parasite abundance per host.
The interaction between connectivity and parasite introduction influenced the peak total number of parasites in the system (p = 0.0085, model R2 = 0.38), being lower in isolated tanks with low parasite introductions (209 ± 91) compared with all other treatments (dispersed metapopulation: 1412 ± 716; clustered metapopulation: 1376 ± 202; high parasite isolated tank: 1822 ± 486). Both the interaction of connectivity and parasite introduction (p = 0.02) and connectivity alone (p = 0.04) influenced peak mean abundance (model R2 = 0.55). Isolation lowered parasite peak mean abundance at low parasite introductions, but increased it at high parasite introductions: the highest mean abundance was observed in high parasite introduction isolated tanks (270 ± 83), followed by high/clustered (71.4 ± 17.8) and low/dispersed (75.3 ± 45) metapopulations, which did not significantly differ, and finally low parasite introduction isolated tanks (33.7 ± 14.4) (figure 3).
4. Discussion
Consistent with our hypothesis, parasites persisted longer in metapopulations than isolated tanks. These results are also consistent with basic metapopulation [4,37,38] and epidemiological theory [7,39,40] as well as predictive models of parasites within a metapopulation context [9,41]. However, to our knowledge, this study is the first to demonstrate this pattern experimentally in vertebrate hosts, in a setting where infection levels on all individuals and subpopulations were quantified and tracked.
Contrary to our expectations, initial parasite distribution did not impact parasite persistence in our metapopulations. We hypothesized that focal introduction of the infection into a single subpopulation would prolong persistence by forcing asynchrony in epidemic dynamics among tanks [14,42–44], leading to higher numbers of susceptible hosts in the metapopulation at any given time (compared with a metapopulation with initially dispersed parasites, where hosts would be expected to transition from infected to refractory in a more synchronous manner). However, we did not observe significant differences in parasite persistence between our clustered and dispersed metapopulations. Although we observed asynchrony during the first 30 days in the clustered metapopulations, thereafter parasite variance to mean ratios among tanks were similar, which might explain why parasite persistence did not differ. Furthermore, Gyrodactylus are often aggregated on individual hosts within the population [45], which in itself is a type of within-population asynchrony in parasite dynamics that could have obscured an effect of asynchrony on persistence. We also observed that aggregation among tanks within metapopulations established shortly after introductions, even when the original parasite introductions were dispersed. Therefore, the only real factor influencing how long gyrodactylids could be sustained was available resources, in this case the number of potentially susceptible hosts to which they had access. Although parasite introduction did not impact persistence within metapopulations, it had a major impact in isolated tanks and on other parameters.
Host mortality and parasite peak loads were higher when more parasites were introduced into a single tank. It makes sense that introducing a higher number of parasites into the tank would lead to higher numbers of parasites per fish and host death, because higher parasite loads increase likelihood of mortality [15,27,46,47]. Consistent with this hypothesis, more fish died in both high parasite isolated tanks and clustered metapopulations compared with those in low parasite isolated tanks. Parasite peak was lowest in low parasite isolated tanks but did not differ among our other three treatments despite differences in connectivity or introduction. In response to Gyrodactylus sp. infection, fish exhibit both a physical response of mucus production [48], which is thought to cause parasite shedding [49] and a non-specific complement that kills gyrodactylids [16,50–52]. It is possible that if the parasite initial load is high, parasite numbers will increase to fatal levels before the fish immune system responds, whereas at low numbers, the fish has time to mount a reaction and slow/eventually stop parasite population growth, as response time can vary under different conditions [53], and ability to resist parasites can vary among individuals based both on genetic background and past exposure [34,46,51,54,55]. Our results indicate that high initial infection leads to worse host outcomes overall, because the two treatments in which high numbers of parasites were introduced experienced the higher mortality and parasite loads compared to treatments where parasites were introduced at low levels. At these high burdens, we also saw that connectivity modulated these effects.
Perhaps our most intriguing result is that the impact of connectivity differed depending on initial parasite burden: when parasites were introduced at low levels, being in a metapopulation helped the parasite (persists longer) but had little/no effect on host outcomes (parasite burden). However, when introduced at high levels, being in a metapopulation also benefitted he hosts, because they had lower parasite burdens than when the parasites were unable to disperse. Peak parasite load and mean abundance were influenced by the interaction of connectivity and level of parasite introduction, with isolation lowering peak parasite loads per fish when parasites were introduced at low levels but increasing them when parasites were introduced at high levels. We expected, and previous studies in similar systems have shown, that the number of available hosts significantly impacts the parasite population size [56]. However, peak parasite numbers were equally high in our clustered metapopulations, dispersed metapopulations and high introduction isolated tanks (which all began with the same total number of parasites), while the number of potential hosts (connectivity) influenced the mean parasite load per fish. Mean abundance was highest when high numbers of parasites were introduced to isolated tanks, and lowest when low numbers were introduced to isolated tanks. In clustered metapopulations, the ability to move and spread parasites to other/naive fish lowered the average burden per host. However, no difference in peak burden was observed between low-parasite isolated tanks and dispersed metapopulations, both of which began with two parasites per tank. This result highlights the importance of studying both hosts and parasites in a metapopulation context.
We identify several strengths and limitations of this study. To the best of our knowledge, this study is the first to experimentally test the impacts of connectivity and initial parasite distribution on vertebrate–parasite metapopulation dynamics. We also conducted four replicates of simulated metapopulation treatments that showed similar and repeatable dynamics. One potential limitation is that dispersal was controlled experimentally, and despite efforts to haphazardly select the dispersing fish, differences in behaviour (shyness/boldness) or health (sicker fish may be slower and therefore easier to catch) may have influenced which fish was selected. However, it has been shown in the wild that sicker fish may also be the ones more likely to disperse downstream [27]. Another potential limitation is that we used laboratory, rather than wild-caught fish and parasites for this experiment. Both fish resistance and parasite virulence are likely to differ in laboratory compared with wild populations, given wide variability in resistance that has been reported among wild populations [20,34], presumably due to different selective pressures [47]. However, our purpose was to provide a general model for this host–parasite system, rather than to directly compare our results with any specific wild population.
This experiment provided laboratory-based evidence that both hosts and parasites can coexist in and benefit from a metapopulation setting, and that the initial distribution of parasites among subpopulations in a metapopulation may determine whether the hosts benefit from connectivity. This finding is of particular importance when species conservation and disease management collide: conservation often emphasizes use of corridors to facilitate species persistence over a patchy landscape, whereas disease management often focuses on transmission interruption through methods such as quarantine [5]. It is also important to recognize that environmental conditions [57,58], heterogeneity among individuals [29,32,46] and other factors may influence disease spread throughout metapopulations. Further investigation under a wider range of conditions and with a diversity of host–parasite systems will be useful in better informing management practices.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Stanley King for identifying the species of Gyrodactylus, and Mark Romer and Claire Cooney for assisting with administration and maintenance of our facilities.
Ethics
Approval for animal care and research was obtained from the McGill University Animal Care Committee (AUP 2014-7547) in compliance with the Canadian Council on Animal Care.
Data accessibility
The primary dataset supporting this manuscript and a short readme file explaining each column is available in Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1d9r423 [59].
Authors' contributions
All authors contributed to the design of the study. C.P.T. collected and analysed data, and wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
Funding for this research was provided by an FQRNT Equipe grant (M.E.S. and G.F.F.) as well as NSERC Discovery grants awarded to M.E.S. and G.F.F. Funding for C.P.T. was provided by an NSERC Alexander Graham Bell Canada Graduate Scholarship—Doctoral Program.
References
- 1.Scott ME. 1988. The impact of infection and disease on animal populations: implications for conservation biology. Conserv. Biol. 2, 40–56. ( 10.1111/j.1523-1739.1988.tb00334.x) [DOI] [Google Scholar]
- 2.Sih A, Jonsson BG, Luikart G. 2000. Habitat loss: ecological, evolutionary and genetic consequences. Trends Ecol. Evol. 15, 132–134. ( 10.1016/S0169-5347(99)01799-1) [DOI] [Google Scholar]
- 3.Huffaker CB, Kennett C. 1956. Experimental studies on predation: predation and cyclamen-mite populations on strawberries in California. Hilgardia. 26, 191–222. ( 10.3733/hilg.v26n04p191) [DOI] [Google Scholar]
- 4.Hanski I. 1999. Metapopulation ecology. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Hess G. 1996. Disease in metapopulation models: Implications for conservation. Ecology. 77, 1617–1632. ( 10.2307/2265556) [DOI] [Google Scholar]
- 6.Bush AO, Kennedy CR. 1994. Host fragmentation and helminth parasites: hedging your bets against extinction. Int. J. Parasitol. 24, 1333–1343. ( 10.1016/0020-7519(94)90199-6) [DOI] [PubMed] [Google Scholar]
- 7.Grenfell B, Harwood J. 1997. (Meta)population dynamics of infectious diseases. Trends Ecol. Evol. 12, 395–399. ( 10.1016/S0169-5347(97)01174-9) [DOI] [PubMed] [Google Scholar]
- 8.McCallum H. 2010. Disease, habitat fragmentation and conservation. Proc. R. Soc. B 269, 2041–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ram K, Preisser EL, Gruner DS, Strong DR. 2008. Metapopulation dynamics override local limits on long-term parasite persistence. Ecology 89, 3290–3297. ( 10.1890/08-0228.1) [DOI] [PubMed] [Google Scholar]
- 10.Keeling MJ. 2000. Metapopulation moments: coupling, stochasticity and persistence. J. Anim. Ecol. 69, 725–736. ( 10.1046/j.1365-2656.2000.00430.x) [DOI] [PubMed] [Google Scholar]
- 11.Thrall PH, Antonovics J. 1995. Theoretical and empirical studies of metapopulations: population and genetic dynamics of the Silene-Ustilago system. Can. J. Bot. 73, 1249–1258. ( 10.1139/b95-385) [DOI] [Google Scholar]
- 12.Thrall PH, Burdon JJ. 2000. Effect of resistance variation in a natural plant host–pathogen metapopulation on disease dynamics. Plant Pathol. 49, 767–773. ( 10.1046/j.1365-3059.2000.00523.x) [DOI] [Google Scholar]
- 13.Holyoak M, Sharon PL. 1996. Persistence of an extinction-prone predator–prey interaction through metapopulation dynamics. Ecology 77, 1867–1879. ( 10.2307/2265790) [DOI] [Google Scholar]
- 14.Jesse M, Ezanno P, Davis S, Heesterbeek JAP. 2008. A fully coupled, mechanistic model for infectious disease dynamics in a metapopulation: movement and epidemic duration. J. Theor. Biol. 254, 331–338. ( 10.1016/j.jtbi.2008.05.038) [DOI] [PubMed] [Google Scholar]
- 15.Scott ME. 1985. Experimental epidemiology of Gyrodactylus bullatarudis (Monogenea) on guppies (Poecilia reticuata): short- and long-term studies. In Ecology and genetics of host-parasite interactions (eds Rollinson D, Anderson RM), pp. 21–38. New York, NY: Academic Press. [Google Scholar]
- 16.Bakke TA, Cable J, Harris PD. 2007. The biology of gyrodactylid monogeneans: the ‘Russian-doll killers'. In Advances in parasitology 64 (eds Baker RM, Rollinson D), pp. 161–376. New York, NY: Academic Press. [DOI] [PubMed] [Google Scholar]
- 17.Cable J, Scott ECG, Tinsley RC, Harris PD. 2002. Behavior favoring transmission in the viviparous monogenean Gyrodactylus turnbulli. J. Parasitol. 88, 183–184. ( 10.1645/0022-3395(2002)088%5B0183:BFTITV%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 18.Johnsen BO. 1978. The effect of an attack by the parasite Gyrodactylus salaris on the population of salmon parr in the river Lakselva, Misvaer in northern Norway. J. Arct. Biol. 11, 7–9. [Google Scholar]
- 19.Johnsen BO, Jenser AJ. 1991. The Gyrodactylus story in Norway. Aquaculture 98, 289–302. ( 10.1016/0044-8486(91)90393-L) [DOI] [Google Scholar]
- 20.Van Oosterhout C, Harris PD, Cable J. 2003. Marked variation in parasite resistance between two wild populations of the Trinidadian guppy, Poecilia reticulata (Pisces: Poeciliidae). Biol. J. Linnean Soc. 79, 645–651. ( 10.1046/j.1095-8312.2003.00203.x) [DOI] [Google Scholar]
- 21.Scott ME, Anderson RM. 1984. The population dynamics of Gyrodactylus bullatarudis (Monogenea) within laboratory populations of the fish host Poecilia reticulata. Parasitology 89, 159–194. ( 10.1017/S0031182000001207) [DOI] [PubMed] [Google Scholar]
- 22.Scott ME. 1985. Dynamics of challenge infections of Gyrodactylus bullatarudis Turnbull (Monogenea) on guppies, Poecilia reticulata (Peters). J. Fish Dis. 8, 495–503. ( 10.1111/j.1365-2761.1985.tb00964.x) [DOI] [Google Scholar]
- 23.Scott ME, Robinson MA. 1984. Challenge infections of Gyrodactylus bullatarudis (Monogenea) on guppies, Poecilia reticulata (Peters), following treatment. J. Fish Biol. 24, 581–586. ( 10.1111/j.1095-8649.1984.tb04828.x) [DOI] [Google Scholar]
- 24.Richards GR, Chubb JC. 1998. Longer-term population dynamics of Gyrodactylus bullatarudis and G. turnbulli (Monogenea) on adult guppies Poecilia reticulata in 50-l experimental arenas. Parasitol. Res. 84, 753–756. ( 10.1007/s004360050481) [DOI] [PubMed] [Google Scholar]
- 25.Harris PD, Lyles AM. 1992. Infections of Gyrodactylus bullatarudis and Gyrodactylus turnbulli on guppies (Poecilia reticulata) in Trinidad. J. Parasitol. 78, 912–914. ( 10.2307/3283329) [DOI] [PubMed] [Google Scholar]
- 26.Johnson MB, Lafferty KD, van Oosterhout C, Cable J. 2011. Parasite transmission in social interacting hosts: monogenean epidemics in guppies. PLoS ONE 6, e22634 ( 10.1371/journal.pone.0022634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Oosterhout C, Mohammed RS, Hansen H, Archard GA, McMullan M, Weese DJ, Cable J. 2007. Selection by parasites in spate conditions in wild Trinidadian guppies (Poecilia reticulata). Int. J. Parasitol. 37, 805–812. ( 10.1016/j.ijpara.2006.12.016) [DOI] [PubMed] [Google Scholar]
- 28.Madhavi R, Anderson RM. 1985. Variability in the susceptibility of the fish host, Poecilia reticulata, to infection with Gyrodactylus bullatarudis (Monogenea). Parasitology 91, 531–544. ( 10.1017/S0031182000062776) [DOI] [PubMed] [Google Scholar]
- 29.Tadiri C, Scott M, Fussmann G. 2016. Impact of host sex and group composition on parasite dynamics in experimental populations. Parasitology 143, 523–531. ( 10.1017/S0031182016000172) [DOI] [PubMed] [Google Scholar]
- 30.Croft D, Arrowsmith B, Bielby J, Skinner K, White E, Couzin I, Magurran AE, Ramnarine I, Krause J. 2003. Mechanisms underlying shoal composition in the Trinidadian guppy, Poecilia reticulata. Oikos 100, 429–438. ( 10.1034/j.1600-0706.2003.12023.x) [DOI] [Google Scholar]
- 31.Kaiser H, Paulet TG, Endemann F. 1998. Water quality fluctuations in a closed recirculating system for the intensive culture of the guppy, Poecilia reticulata (Peters). Aquacult. Res. 29, 611–615. ( 10.1111/j.1365-2109.1998.tb01176.x) [DOI] [Google Scholar]
- 32.Tadiri CP, Dargent F, Scott ME. 2012. Relative host body condition and food availability influence epidemic dynamics: a Poecilia reticulata–Gyrodactylus turnbulli host–parasite model. Parasitology 140, 1–9. [DOI] [PubMed] [Google Scholar]
- 33.Schelkle B, Doetjes R, Cable J. 2011. The salt myth revealed: treatment of gyrodactylid infections on ornamental guppies, Poecilia reticulata. Aquaculture 311, 74–79. ( 10.1016/j.aquaculture.2010.11.036) [DOI] [Google Scholar]
- 34.Cable J, van Oosterhout C. 2007. The role of innate and acquired resistance in two natural populations of guppies (Poecilia reticulata) infected with the ectoparasite Gyrodactylus turnbulli. Biol. J. Linnean Soc. 90, 647–655. ( 10.1111/j.1095-8312.2006.00755.x) [DOI] [Google Scholar]
- 35.Scott ME. 1982. Reproductive potential of Gyrodactylus bullatarudis (Monogenea) on guppies (Poecilia reticulata). Parasitology 85, 217–236. ( 10.1017/S0031182000055207) [DOI] [Google Scholar]
- 36.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 37.Hanski I. 1991. Single-species metapopulation dynamics: concepts, models and observations. Biol. J. Linnean Soc. 42, 17–38. ( 10.1111/j.1095-8312.1991.tb00549.x) [DOI] [Google Scholar]
- 38.Hanski I, Ovaskainen O. 2003. Metapopulation theory for fragmented landscapes. Theor. Popul. Biol. 64, 119–127. ( 10.1016/S0040-5809(03)00022-4) [DOI] [PubMed] [Google Scholar]
- 39.Hagenaars TJ, Donnelly CA, Ferguson NM. 2004. Spatial heterogeneity and the persistence of infectious diseases. J. Theor. Biol. 229, 349–359. ( 10.1016/j.jtbi.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 40.Naug D. 2010. Disease transmission and networks. In Encyclopedia of animal behavior (eds Breed MD, Moore J), pp. 532–536. Oxford, UK: Academic Press. [Google Scholar]
- 41.Colizza V, Vespignani A. 2008. Epidemic modeling in metapopulation systems with heterogeneous coupling pattern: theory and simulations. J. Theor. Biol. 251, 450–467. ( 10.1016/j.jtbi.2007.11.028) [DOI] [PubMed] [Google Scholar]
- 42.Earn DJ, Rohani P, Grenfell BT. 1998. Persistence, chaos and synchrony in ecology and epidemiology. Proc. R. Soc. Lond. B 265, 7–10. ( 10.1098/rspb.1998.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lloyd AL, Jansen VA. 2004. Spatiotemporal dynamics of epidemics: synchrony in metapopulation models. Math. Biosci. 188, 1–16. ( 10.1016/j.mbs.2003.09.003) [DOI] [PubMed] [Google Scholar]
- 44.Rohani P, Earn DJD, Grenfell BT. 1999. Opposite patterns of synchrony in sympatric disease metapopulations. Science 286, 968–971. ( 10.1126/science.286.5441.968) [DOI] [PubMed] [Google Scholar]
- 45.Scott ME. 1987. Temporal changes in aggregation: a laboratory study. Parasitology 94, 583–595. ( 10.1017/S0031182000055918) [DOI] [PubMed] [Google Scholar]
- 46.Dargent F, Scott ME, Hendry AP, Fussmann GF. 2013. Experimental elimination of parasites in nature leads to the evolution of increased resistance in hosts. Proc. R. Soc. B 280, 2013–2371. ( 10.1098/rspb.2013.2371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cable J, van Oosterhout C. 2007. The impact of parasites on the life history evolution of guppies (Poecilia reticulata): the effects of host size on parasite virulence. Int. J. Parasitol. 37, 1449–1458. ( 10.1016/j.ijpara.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 48.Gheorghiu C, Cable J, Marcogliese DJ, Scott ME. 2007. Effects of waterborne zinc on reproduction, survival and morphometrics of Gyrodactylus turnbulli (Monogenea) on guppies (Poecilia reticulata). Int. J. Parasitol. 37, 375–381. ( 10.1016/j.ijpara.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 49.Lester RJG. 1972. Attachment of gyrodactylus to gasterosteus and host response. J. Parasitol. 58, 717–722. ( 10.2307/3278299) [DOI] [PubMed] [Google Scholar]
- 50.Woo PT. 2006. Fish diseases and disorders, 2nd edn Oxfordshire, UK: CABI Publishing. [Google Scholar]
- 51.Sato A, figueroa F, O'Huigin C, Reznick DN, Klein J. 1995. Identification of major histocompatibility complex genes in the guppy, Poecilia reticulata. Immunogenetics 43, 38–49. ( 10.1007/BF00186602) [DOI] [PubMed] [Google Scholar]
- 52.Robertson S, Bradley JE, MacColl ADC. 2017. No evidence of local adaptation of immune responses to Gyrodactylus in three-spined stickleback (Gasterosteus aculeatus). Fish Shellfish Immunol. 60, 275–281. ( 10.1016/j.fsi.2016.11.058) [DOI] [PubMed] [Google Scholar]
- 53.Gheorghiu C, Marcogliese DJ, Scott ME. 2009. Temporal dynamics of epidermal responses of guppies Poecilia reticulata to a sublethal range of waterborne zinc concentrations. J. Fish Biol. 75, 2642–2656. ( 10.1111/j.1095-8649.2009.02457.x) [DOI] [PubMed] [Google Scholar]
- 54.Fraser B, Neff B. 2010. Parasite mediated homogenizing selection at the MHC in guppies. Genetica 138, 273–278. ( 10.1007/s10709-009-9402-y) [DOI] [PubMed] [Google Scholar]
- 55.Fraser BA, Ramnarine IW, Neff BD. 2009. Selection at the MHC class IIB locus across guppy (Poecilia reticulata) populations. Heredity 104, 155–167. ( 10.1038/hdy.2009.99) [DOI] [PubMed] [Google Scholar]
- 56.Bagge AM, Poulin R, Valtonen ET. 2004. Fish population size, and not density, as the determining factor of parasite infection: a case study. Parasitology 128, 305–313. ( 10.1017/S0031182003004566) [DOI] [PubMed] [Google Scholar]
- 57.Acevedo-Whitehouse K, Duffus ALJ. 2009. Effects of environmental change on wildlife health. Phil. Trans. R. Soc. B 364, 3429–3438. ( 10.1098/rstb.2009.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gheorghiu C, Marcogliese DJ, Scott M. 2006. Concentration-dependent effects of waterborne zinc on population dynamics of Gyrodactylus turnbulli (Monogenea) on isolated guppies (Poecilia reticulata). Parasitology 132, 225–232. ( 10.1017/S003118200500898X) [DOI] [PubMed] [Google Scholar]
- 59.Tadiri CP, Scott ME, Fussmann GF. 2018. Data from: Microparasite dispersal in metapopulations: a boon or bane to the host population? Dryad Digital Repository. ( 10.5061/dryad.1d9r423) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tadiri CP, Scott ME, Fussmann GF. 2018. Data from: Microparasite dispersal in metapopulations: a boon or bane to the host population? Dryad Digital Repository. ( 10.5061/dryad.1d9r423) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The primary dataset supporting this manuscript and a short readme file explaining each column is available in Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1d9r423 [59].