Abstract
Precision cerebrovascular health or individualized long-term preservation of the brain and associated blood vessels, is predicated on understanding, diagnosing, and tailoring therapies for people at risk of ischemic injury associated with stroke and vascular dementia. The associated imaging patterns are sculpted by the protective effect of the collaterome, the innate compensatory ability of the brain and vasculature to offset hypoperfusion when antegrade or normal arterial inflow pathways are compromised. Theranostics or rational and synchronous use of diagnostic studies in tandem with specific therapies to optimally guide patient outcomes in ischemic brain disorders may capitalize on the pivotal role of the collaterome. Understanding the functional impact of the collaterome across populations of individuals would advance translational science on the brain, while questions with immediate clinical implications may be prioritized. Big data and systematic analyses are necessary to develop normative standards, multimodal imaging atlases, and delineation of specific patterns to guide clinical management. Large-scale, systematic imaging analyses of the collaterome provide a platform for translational work on cerebral collateral circulation and hemodynamics and a theranostic framework with direct clinical implications. This article frames incipient research objectives to guide precision stroke medicine in coming years, building upon the collaterome concept in brain health.
Keywords: Collaterals, precision, theranostics, cerebral ischemia, collaterome, stroke, dementia, imaging
Introduction
Precision cerebrovascular health or individualized long-term preservation of the brain and associated blood vessels, is predicated on understanding, diagnosing, and tailoring therapies for people at risk of ischemic injury associated with stroke and vascular dementia.1,2 The diverse spectrum of ischemia, or depravation of nutritive blood flow to the brain, has been traditionally and archaically split into seemingly distinct diagnoses of acute stroke due to myriad etiologies, silent stroke and dementia, or vascular cognitive impairment.3 The pattern of ischemia and associated hypoperfusion evident on imaging of these various disorders may be leveraged to optimize management of such individuals at risk.4 Such profiles or imaging patterns are sculpted by the protective effect of the collaterome, the innate compensatory ability of the brain and vasculature to offset hypoperfusion when antegrade or normal arterial inflow pathways are compromised.4–7 Although specific collateral flow routes are readily identified, the detailed characterization of the collaterome, including the associated functional and metabolic correlates of particular blood flow patterns has been surveyed only superficially to date.4 Theranostics or the rational and synchronous use of diagnostic studies in tandem with specific therapies to optimally guide patient outcomes, in the setting of ischemic brain disorders including stroke and dementia may capitalize on the pivotal role of the collaterome.8,9 A wide array of neurological disorders, from silent stroke to transient ischemic attacks, stroke and vascular cognitive impairment, all invoke ischemia and the balancing effects of various collateral circulatory patterns.3 These diagnoses have been routinely separated and not approached from such a thematic or holistic perspective where the collaterome is so influential.5–7 The ensuing review of these disorders and the potential therapeutic strategies hinge on the hypothesis that even routine imaging patterns may be used to redefine the impact of commonly prescribed strategies for patients at risk of ischemic stroke and vascular dementia, focusing on the pivotal role of the collaterome. Considerable effort has been devoted to develop novel medications and devices for cerebral ischemic disorders, yet understanding and categorizing cases based on the imaging profiles of collateral blood flow patterns may be used to discern the impact of many currently employed or routine interventions.4 A systematic approach and focus on the hemodynamics evident on imaging studies may be used to classify particular ischemic disorders and hence match specific therapies to hemodynamic patterns.10 This theranostic strategy seems explicit, yet it has not been employed to date. In fact, categorization has been based on arterial lesions without consideration of corresponding collateral perfusion that varies from one individual to the next. In other disorders, such as small vessel disease or vascular dementia where hypoperfusion is a recognized factor, the collateral blood flow patterns have not been detailed.8,11–17
Collaterome
The collaterome mitigates hypoperfusion and cerebral ischemia, the key mechanisms of brain injury in the continuum of stroke and dementia.5–7,9,12 Vascular risk factors are inimitably linked with stroke, yet the overlap with dementia or vascular cognitive impairment underscores the need to discern key pathophysiology that may incur hypoperfusion.8,13–15,18,19 Hypertension is often implicated as the sole culprit in the cerebrovascular injury of stroke and vascular cognitive impairment, yet the regulation of cerebral perfusion and vascular biology of hemodynamics and collateral perfusion are considerably complex. Treating hypertension alone may not address the role of collaterals and hemodynamics in these scenarios. The collaterome or systems biology approach to collateral circulation in the brain is not limited to specific anastomotic connections, but considers the entire constellation of mechanisms that offset potential ischemia.5–7 The collaterome is pivotal in both acute and chronic clinical scenarios where hypoperfusion may lead to ischemia.20 The current discussion considers the imaging aspects of the collaterome without delving into other potential biomarkers of collaterals. Mapping the collaterome with routinely employed imaging modalities may disclose specific patterns to guide management across a wide array of cerebrovascular disorders. Such collateral maps define not just the potential brain vascular supply, but also potential plasticity in the face of ischemia (Figure 1). Although rooted in specific or selected imaging patterns, large-scale and normative data are necessary, akin to the trajectory of conenctomics.21,22 The following topical review provides a modern and futuristic view of how mapping the collaterome and cerebral hemodynamics may delineate optimal strategies to defeat the potentially devastating consequences of hypoperfusion and ischemia for each individual.
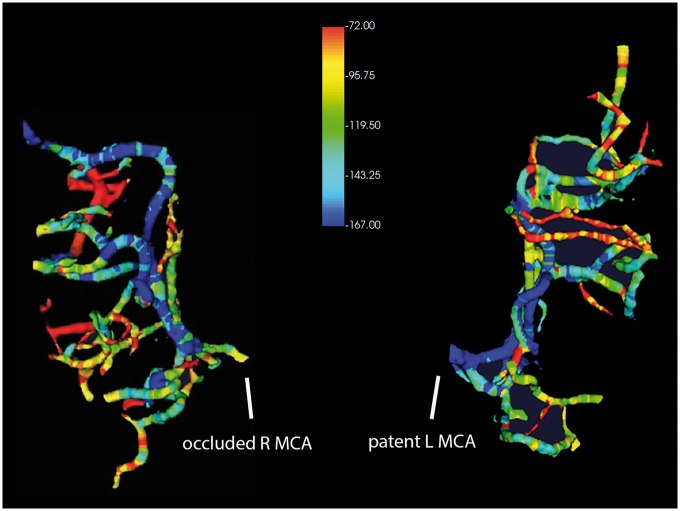
Figure 1.
CTA gradient maps, quantifying the incremental Hounsfield unit changes of an occluded right MCA (left), reveal marked variability in collateral flow across branches of the downstream territory. In comparison, the patent left MCA (right) displays antegrade flow-related gradients. These illustrative images are generated from single-phase CTA of a 70-year-old woman with acute onset of left hemiparesis and neglect.
The concept of the collaterome is an extension of the emerging construct that systems biology or a multitude of diverse mechanisms and cellular elements interact to produce certain biologic responses in the brain.5–7 Similar to the neurovascular unit, the collaterome underscores the need to consider vascular, neuronal and glial interactions in concert. The role of collateral circulation in both acute ischemic stroke and in chronic disorders such as intracranial atherosclerosis or extracranial carotid artery disease has been well established.4,23,24 The focus, however, has been on the status of collateral blood flow routes at one specific time point rather than on the biology or mechanisms involved in the development, maturation and sustenance of these pathways. The lack of systematic, comprehensive evaluation of such collateral circulation over time has limited insight to the appearance of select collateral routes in somewhat anecdotal situations. For instance, the appearance of leptomeningeal or pial collaterals in acute stroke has been instrumental in establishing the role of collateral perfusion, yet little work has been devoted to understanding the nature of such reverse arterial blood flow, biophysical parameters, genomic or exomic correlates and corresponding metabolic events. As a result, even the most groundbreaking advances in the lab, discerning the genomic underpinnings of pial collateral status, hit an impasse in translation to clinically relevant scenarios in humans.25 Methodical categorization of collateral blood flow patterns in humans from acute stroke to chronic scenarios is therefore necessary. Mapping the collaterome is most easily achieved with imaging the topography or distribution of blood flow in common cerebrovascular disorders. Although collateral circulation to the brain has been traditionally divided into Willisian routes at the Circle of Willis and more downstream perfusion channels via the leptomeningeal anastomoses, it remains most likely that the critical patterns of hypoperfusion are determined by the most distal arterial branches and associated venous changes (Figure 2).26 Most research has centered on the links or anastomoses, rather than on the downstream elements contributing to perfusion of a specific brain region. For example, the ultimate cortical distribution and spatial topography of an infarct downstream from a proximal middle cerebral artery (MCA) occlusion are largely due to the local blood flow environment of a distal MCA segment receiving blood flow in reverse direction. The status of specific pial anastomoses delivering blood flow from the adjacent anterior or posterior cerebral arteries (ACA or PCA) may not be as important.
Figure 2.
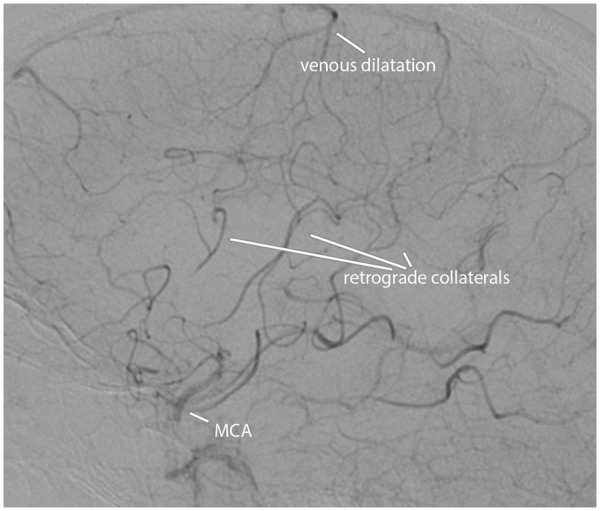
Leptomeningeal arterial collaterals and associated venous dilatation downstream from an occluded left MCA, demonstrated on a lateral projection of DSA in a 56-year-old woman with sudden onset of right hemiparesis.
The specifics of collateral supply to the brain are mostly limited to identification of certain routes, with only superficial understanding of how the most downstream collateral blood flow routes are regulated or influenced. From a clinical perspective, the study of collaterals and the collaterome has been severely limited by numerous factors. Rather than endorsing the rudimentary fact that ischemia is caused by hypoperfusion modulated by the collaterome, we approach collateral flow from very different perspectives in each clinical scenario. In acute stroke, collateral status may be grossly categorized as “good” or “poor” on noninvasive imaging studies such as CTA or MRA. Even when digital subtraction angiography (DSA) is performed in the setting of endovascular therapy, collateral status is grossly subdivided into partial or complete and delayed or rapid categories, rather than investigating the nature of such reverse arterial blood flow to a specific area of the brain. In more chronic conditions, such as carotid disease or moyamoya syndrome, the collaterome is characterized solely by rough perfusion patterns or the presence of certain Willisian routes without investigation of distal collateral physiology.27 Even more so, in other ischemic conditions such as small vessel disease or vascular dementia, hypoperfusion is implicated, yet there is almost no investigation of the collaterome.8,14–17 Such disregard of the collaterome has been fueled by longstanding debate on the existence or lack thereof in subcortical collaterals. Lenticulostriate and other deep arterial perforators have been cast as end arteries, devoid of collaterals, yet conditions such as moyamoya syndrome reveal the vast collateral potential to completely offset subcortical ischemia (Figure 3). When diffuse small vessel disease is considered, almost no attention has been given to perfusion patterns and the local microfluidic or blood-brain barrier changes, whereas intrinsic end artery disease is blamed.16,17 In all these scenarios, mapping the collaterome or constructing an atlas of collateral systems may provide insight on the underlying pathophysiology and directed treatment of specific patterns.
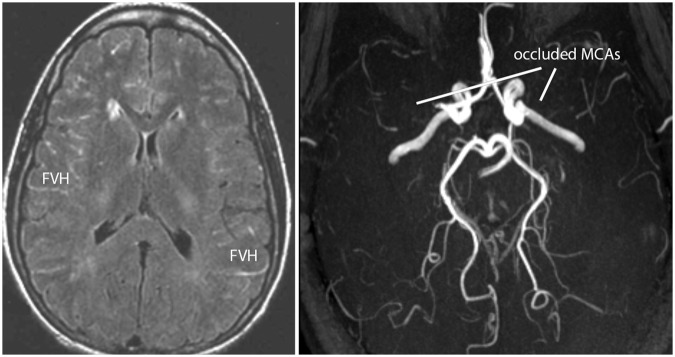
Figure 3.
FLAIR MRI (left) reveals FLAIR vascular hyperintensities (FVH) and paucity of subcortical ischemic injury in the setting of bilateral MCA occlusions on MRA (right) associated with moyamoya syndrome in a 28-year-old woman with frequent headaches and a recent transient ischemic attack presenting with right hemisensory loss.
Rationale for collateromics
Recent endorsements for understanding brain structure, function, and plasticity as in connectomics have not addressed the dynamic and pivotal role of collaterals in vascular disorders. Imaging advances in brain structure with diffusion-tensor imaging and functional mapping with BOLD techniques have fueled connectomics.28,29 Other imaging advances are effectively remapping the traditional architecture of the brain, providing innumerable opportunities for progress in neurological disorders. Akin to connectomics and high-resolution structural imaging of the brain, collateromics offers a unique trajectory to understand hypoperfusion, ischemia, and vascular adaptation across acute and more chronic disorders in the brain. Unlike connectomics, where direct connections are often mapped through subcortical pathways, the study of collateromics entails more indirect and circuitous vascular relationships spanning the brain surface. The study of these vascular networks, variability across individuals within a population and their dynamic response to changes in cerebral blood flow has received scant attention to date. Cerebrovascular health may be transformed by such comprehensive investigation of collateromics, revealing serial changes in perfusion in response to a variety of arterial disorders affecting the brain, from extracranial large vessel disease to diffuse microvascular or small vessel disease.
Collateromics is driven by the need to understand the causes, topography and homeodynamic facets of hypoperfusion. The clinical impact of collateromics in stroke is unequivocal, whereas the role of hypoperfusion in dementia and potential of collateral blood flow is less well defined.9 In most circumstances, our observations are limited to isolated snapshots or relatively static imaging of the brain without an understanding of the evolution of such patterns over time. Most recently, the basic science of collaterals has rapidly advanced, yet the most rational translational steps into the clinical paradigm remain obscure due to relative inattention regarding collateral imaging.25,30–33 The longitudinal changes in blood flow or collateromics over years and months after an initial ischemic episode also remain limited due to the paucity of serial blood flow imaging in clinical routine. Ultimately, mapping the collaterome with even the most rudimentary imaging data would provide a translational platform to advance basic science of collaterals, extrapolation into the clinical realm, and tailored management of individuals at risk of both stroke and dementia.25,31–33 Routine imaging of individuals with ischemia is readily available, even if it reduces to the most basic consideration of infarct patterns on noncontrast computed tomography. Evaluation of serial imaging, acquired at different time points, provides a perspective that may prompt additional pursuit of perfusion or noninvasive angiographic imaging correlates. The temporal evolution of hypoperfusion and associated imaging correlates provides a novel approach to long-term management of ischemia, rather than the current episodic paradigm where all efforts are pinned on delineating extent of the ischemic penumbra. In sum, the entire imaging focus currently revolves around determination of acute ischemia while neglecting the chronic component of hypoperfusion that may be more difficult to ascertain. The fluctuating nature of hypoperfusion and associated ischemia is rarely the target of imaging surveillance, whereas extensive attention is placed on measuring ischemic parenchymal lesions or degree of luminal stenosis in feeding arteries to the brain.
Collateromics will prevail once the continuum of ischemia is realized with imaging, from the most transient episodes, to silent lesion evolution, acute or overt manifestations as stroke and the more insidious progression of lesion burden associated with vascular cognitive impairment.3 Prevention and the timely intervention to avert such deleterious effects require recognition of hypoperfusion and impetus to target collateromics. Historically, stroke and dementia have been artificially separated in clinical management, despite considerable overlap in vascular pathophysiology.9,13,15 Patients are often segued into seemingly distinct clinics despite the fact that imaging of their brains reveals commonalities in hypoperfusion. Most recently, recognition of vascular correlates in a variety of dementia types has been re-emphasized.8,18 Imaging patterns and associated collateromics may reveal important overlap in pathophysiology, unrecognized at the clinical level alone without insight from imaging.15 Even in acute stroke, the overwhelming focus has been on arterial occlusions or stenoses with minimal consideration of collaterals and compensatory factors to ischemia. In more chronic scenarios, such as intracranial atherosclerosis or small vessel disease, hypoperfusion exists without concerted research or routine clinical investigations.16,17 Similarly, the evaluation and management of extracranial carotid artery disease have continued to center on the occlusive lesion in the neck despite much evidence that hypoperfusion may be instrumental.27 The relevance of the collaterome in regard to clinical research in both acute and chronic cerebral ischemia is best exemplified by detailed analyses of recent clinical trials. In acute ischemic stroke, a litany of landmark endovascular trials of mechanical thrombectomy culminated in successful results, largely in part due to the consideration of collateral status gleaned from imaging. A variety of imaging selection approaches for potential revascularization candidates were used, including ASPECTS scores on non-contrast CT, perfusion imaging, and diverse techniques for CT angiography (CTA). All of these selection paradigms focused on inclusion of those individuals with more robust collateral status and avoidance of those with poor or marginal collaterals. Not only were these trials terminated early for success based on much smaller sample sizes than originally powered, subsequent post hoc analyses of these datasets revealed that further distinction of collateral grades within these populations was influential with respect to clinical outcomes. The CTA analyses of collaterals in the MR CLEAN trial provides an excellent example of this saga.34 In more chronic disorders, such as intracranial atherosclerosis, the WASID and SAMMPRIS trials focused on the degree of arterial stenosis rather than the functional correlates such as hemodynamic impact, collaterals, or precise identification of individually vulnerable subjects.23,24,35 Collateral status proved to be the most influential factor in determining subsequent clinical outcomes.23 Paradoxically, such insight in both acute and chronic scenarios has not been translated into subsequent clinical trial designs, largely because of impracticality in rapidly and reliably discerning collateral grade in routine practice or local investigator enrollment for a trial. Mapping the collaterome and the development of practical tools to automate collateral grade would undoubtedly facilitate future paradigms that incorporate, rather than ignore such pivotal pathophysiology. Recognition of hypoperfusion as the key precipitant of ischemia in most of these clinical scenarios emphasizes the need to map and characterize the nature of collaterals and large-scale study of these vascular networks across populations at various stages of health and disease.
Phenotypying for precision
The imaging-defined phenotype of lesion patterns and associated hypoperfusion provides a rational basis for precision cerebrovascular health.2,36 This precision phenotype of cerebral ischemia contrasts with the intense emphasis on genomics that fueled precision medicine across many other conditions.1,37 In cerebral ischemia, the individual manifestations of collateral circulation distinguish seemingly homogenous conditions such as proximal MCA occlusion.38 From one individual to the next, the heterogeneity and topography of collateral perfusion to downstream cortical regions are distinct, providing a virtual fingerprint or signature of disease that may be leveraged to define specific therapeutic strategies (Figure 4). At present, the most detailed investigations of collaterals in acute stroke ascribe a singular grade to the collateral filling pattern of the entire territory. Each patient may be described by a single collateral grade, despite marked differences in cortical hypoperfusion from one brain region to the next. Almost half of all acute ischemic stroke patients manifest partial collateral filling or an intermediary grade that itself encompasses a broad range of individual collateral perfusion patterns. Some patients have partial filling of the downstream territory with relatively compromised clinical outcomes after revascularization, whereas other individuals with partial yet more extensive filling of the territory have markedly better outcomes (Figure 5). Even these collateral filling patterns do not address the functional and incredibly important mechanistic features of the downstream microcirculation and tissue perfusion. Novel imaging algorithms, such as post-processing with perfusion angiography (Figure 4), may directly associate such anatomical features of collateral vessels with corresponding perfusion.39 Rapid evaluation of collateral grade is feasible in current clinical practice and will undoubtedly be readily available in future paradigms where mapping the collaterome with perfusion angiography can provide quantitative information on collateral status at angiography. The temporal evolution or timing of such collateral patterns is also remiss from current practice patterns. At present, it remains completely unclear how collateral filling patterns may vary in the first few hours after ischemic onset or whether later depictions of collaterals infer more stable configurations of cerebral blood flow. In the future, rapid and reliable noninvasive quantification of collateral grade may be achieved with noninvasive imaging techniques such as CTA or MRA, yet such methods require validation against the reference standard of DSA. In sum, the presence of a proximal MCA occlusion is a relatively minor contributor to infarct evolution when compared with the collateromics affecting the downstream vasculature and territory. Current therapeutic strategies largely ignore such distinctions and treat all such cases indiscriminately despite marked differences in subsequent outcomes. Other patterns such as subcortical lesions are assumed to be due to intrinsic small vessel disease without consideration of territorial perfusion.13,40 Large subcortical infarcts, periventricular lesions, and many other findings attributed to lacunar disease are routinely blamed on microvascular pathophysiology seemingly unaffected by cerebral hypoperfusion. Even before such advanced disease manifestations become evident, the status of collateral circulation and potential to offset ischemia may be investigated if phenotypes are considered on readily available imaging modalities commonly acquired.38
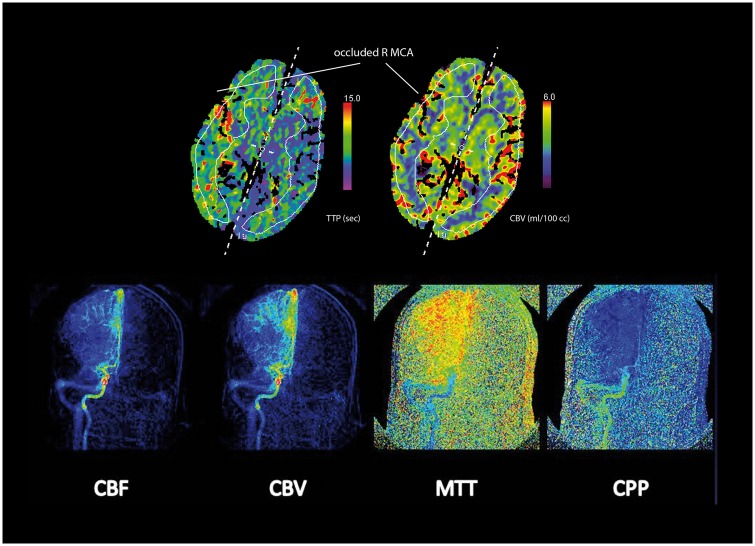
Figure 4.
CT perfusion maps (above) of time-to-peak (TTP) measures in seconds of delay and cerebral blood volume (CBV) in ml/100 cc revealing delayed perfusion via collaterals that have relatively preserved the normal amount of CBV downstream from a right MCA occlusion in a 64-year-old man with left hemiparesis. A heterogeneous pattern of perfusion is noted across different regions of this ischemic territory, due to differences in regional collateral filling. Perfusion angiography images (below) generated from the DSA yields perfusion maps of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and cerebral perfusion pressure (CPP) associated with collateral filling in this right MCA occlusion.
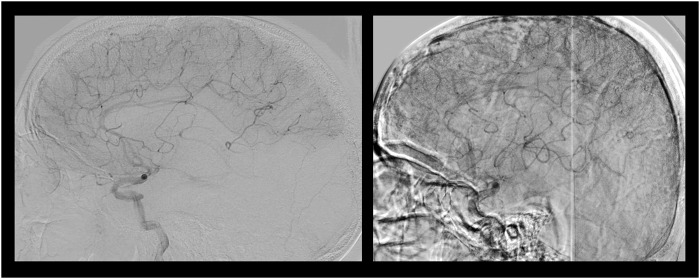
Figure 5.
Lateral projections of DSA in two distinct cases of acute MCA stroke where collateral grade is identical, revealing partial filling of the downstream ischemic territory. The image on the left reveals limited extent of collateral perfusion in a patient with persistent disability at day 90. In contrast, the image on the right depicting more extensive, yet partial filling of the MCA territory was associated with minimal disability or mRS of 1 at day 90 after revascularization.
A map or predictive algorithm of collaterals may help define optimal management of an individual along the continuum of cerebrovascular disease from the earliest stages. Unfortunately, imaging evaluation of the brain and vascular structures is driven by clinical symptoms that infer collateral insufficiency and evolving injury. Collateral recruitment and arteriogenesis may occur long before symptoms prompt imaging evaluation. This paradox has been engrained by somewhat artificial definitions used in clinical practice that distinguish health versus disease and acute from chronic stages of ischemia. Even minor alterations in collateral status may trigger acute symptoms in chronic cerebrovascular conditions, making the distinction between acute and chronic or symptomatic and asymptomatic pathology almost negligible. For instance, dynamic changes in collateral circulation may instantaneously render a chronic, asymptomatic carotid lesion as a medical emergency demanding revascularization for symptomatic disease. Similarly, acute-on-chronic ischemia in moyamoya syndrome may be prompted by a global alteration in cerebral blood flow and complex collateral networks even when focal arterial lesions of the terminal carotid are deemed the culprit lesion. Collateromics and the associated phenotypes evident on imaging may provide an ideal opportunity for prevention of cerebrovascular disease and preservation of brain integrity rather than awaiting overt clinical symptoms.
The phenotype of global or diffuse cerebral hypoperfusion may also provide theranostic clues, despite the traditional mindset that such patterns are unrelated to collaterals. Diminished cardiac output associated with atrial fibrillation and intrinsic vascular derangements of dementia may be modified by innate collateral status.9,41,42 Imaging patterns of diffuse disease evolution such as white matter lesions may be explained by perfusion abnormalities.17,43 Although multiple mechanisms of injury may exist beyond ischemia, imaging patterns of hypoperfusion and collateromics may be gleaned from serial imaging and longitudinal changes.
Historical context
The innovation of mapping the collaterome starkly contrasts with the traditional approach of characterizing collateral networks in only select circumstances. Unlike the highly selective and consequently skewed depiction of collaterals in current practice, systematic mapping and the development of normative standards from available imaging would provide a basis to understand individual distinctions in proper context.21 Current approaches to evaluate individuals with potential cerebrovascular disease typically prioritize imaging acquired at symptomatic presentation, in isolation from available longitudinal changes in the brain on serial imaging. Most individuals at risk of stroke and dementia normally undergo brain imaging on multiple occasions over months, years and decades. The lack of comprehensive imaging datasets, including both stroke and dementia, has hampered our understanding of collateromics and potential theranostic value. Co-registration and numerous post-processing algorithms may leverage the longitudinal information embedded in routinely acquired imaging studies of individuals throughout various cerebrovascular stages, from health to advanced disease. In the future, meta-data from routine imaging may be immediately used to guide therapies when comparative analyses of such post-processing are available from a larger population. Important distinctions in collateral maps may be disclosed, revealing the effects of aging and co-morbidities such as diabetes. The relationship of collateromics with subcortical lesions would complement current connectomic approaches. Additionally, the focus on underlying pathophysiology rather than the effect of specific therapies may reveal novel opportunities to intervene and accelerate the most recent advances from the lab. Most importantly, the prognosis of cerebrovascular alterations would be markedly advanced and stroke epidemiology radically modernized.
Mapping the biology and longitudinal changes of the collaterome
Numerous facets of the collaterome, from the vascular anatomy to the functional impact on regional perfusion, may be atlased with multimodal CT and MRI. Noninvasive angiography and perfusion sequences may easily chronicle the subtlest changes that evolve over time within an individual’s brain. When DSA is available, correlative pathophysiology may also be disclosed. Such detailed evaluation of collateral status and corresponding cerebral blood flow patterns are well established in isolated cases, yet the relative severity or impact is unknown at the population level. In brief, the maximal information gleaned from imaging the collaterome would provide perspective on individual dimensions relative to the larger population and within an individual’s brain over time.
Mapping the collaterome requires a dedicated focus on hypoperfusion and alleviating effects of collateral circulation that may be depicted with myriad imaging modalities, from transcranial Doppler ultrasonography (TCD) to CT angiography (CTA), MR angiography (MRA), DSA, CT perfusion (CTP), dynamic contrast-enhanced perfusion imaging with MRI (PWI) or arterial spin labeled perfusion MRI (ASL).12,44 Cross-modality comparisons or analyses are also informative as they may provide complementary data and individuals often undergo multiple imaging modalities. Unlike the overwhelming emphasis placed on defining the anatomy of arterial lesions such as the degree of stenosis in a proximal vessel, both the structural configuration and functional or hemodynamic dimensions of collaterals must be gauged. This seemingly subtle, yet important consideration of the functional features of collateral perfusion is critical as it has been demonstrated that the presence of collateral vessels or even angiogenic vessels may not deliver equivalent perfusion in downstream tissue.45
Current knowledge on the configuration of the Circle of Willis, leptomeningeal collaterals, and territorial perfusion is based on incredibly small datasets that largely ignore temporal changes over time. For instance, numerous papers have reported conflicting data on the potential protective effect of intact arterial segments at the Circle of Willis. Some studies suggest that complete patency at the Circle of Willis confers protection from subsequent ischemia, whereas others suggest that such a pattern infers risk due to exhausted recruitment of potential collaterals.46,47 Such differences may be better explained by serial imaging of the Willisian segments over time, to depict the impact of longitudinal changes.48 Our knowledge of leptomeningeal collaterals is similarly limited by adequate context over time. Robust collaterals at angiography are associated with smaller infarcts and better outcomes after endovascular therapy for acute ischemic stroke, yet the role of such collaterals with delayed intervention may have radically different impact.38 The downstream collateral perfusion delays often measured on CT or MRI perfusion imaging techniques as Tmax lesions and associated patterns have been equated with ischemic penumbra, inevitably destined to infarction without revascularization. These areas of Tmax abnormalities, or the delayed cerebral perfusion quantified by the time to maximum contrast concentration after bolus injection suggest pathophysiological consequences and any delay is not considered normal. However, when such identical perfusion patterns are visualized in populations that harbor underlying intracranial atherosclerosis, the expected inevitable infarction does not take place, likely because the associated cerebral blood volume or CBV in these regions is adequate to compensate for delayed perfusion. Furthermore, Tmax lesions may persist as chronic and asymptomatic imaging findings, dramatically challenging their equivalence with ischemic penumbra.
Outstanding questions on the collaterome
Understanding the functional impact of the collaterome across populations of individuals with varying degrees of cerebrovascular lesion burden would advance translational science on the brain, while a litany of questions with immediate clinical implications may be prioritized.33 The pace of collateral recruitment and development may be readily discerned, providing insight on the timeline of hemodynamic changes in the brain. Normative maps of the leptomeningeal collateral circulation may answer basic questions regarding the impact of aging and differences based on sex.25 Concomitant environmental or co-morbid conditions such as smoking or the presence of diabetes may be related to arteriogenesis or impaired collateral development. The nature of reverse arterial blood flow via collaterals may be characterized to explain the impact of local pressure gradients and collateral failure. Noninvasive CTA measures of the collaterome may reveal important biophysical parameters that differentiate particular flow routes. Computational fluid dynamics may further disclose microvascular changes that influence cerebral blood flow (Figure 6). Detailed atlases of hypoperfusion in acute stroke may be explained by regional insufficiency of leptomeningeal collaterals. Cortical mapping of the collaterome may provide a logical basis for revascularization options in moyamoya syndrome or a rational approach to incorporate hypoperfusion as a factor in decision-making for carotid revascularization.27 Similarly, the constellation of regional collateral perfusion patterns during endovascular therapy for acute ischemic stroke may be used to stage or dictate interval therapeutic steps. Current questions about collateral augmentation may also be answered, providing novel therapeutic opportunities.10 For instance, vasodilation of leptomeningeal collaterals may be achieved in rodents, whereas the feasibility or impact in humans remains unknown.30,49 The impact of routinely delivered therapies, such as pressors or fluid administration in the intensive care unit, may be critically evaluated with respect to collateral status and blood pressure measures.10,50 At present, the relationship between blood pressure and cerebral blood flow remains mostly unknown. Perhaps most importantly, the underlying nature of collateral networks may be used to resolve current quandaries where higher blood pressures have been associated with lower cerebral blood flow measures.51 The role of hypoperfusion in atrial fibrillation and vascular dementia may be concretely determined and related to variable collateral networks.41,42 Novel observations on metabolic changes and other triggered cascades may be discerned, possibly explaining the inflammatory basis of arteriogenesis in the brain and relationship with blood–brain barrier alterations. None of these advances, however, is possible without systematic, comprehensive mapping of the collaterome.
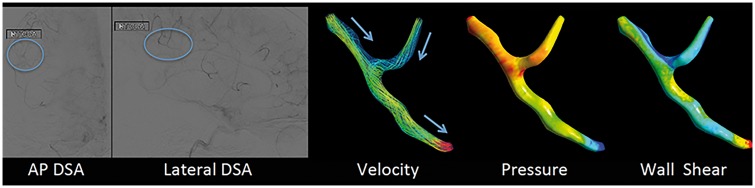
Figure 6.
Computational fluid dynamics (CFD) of retrograde leptomeningeal collateral flow generated from DSA in acute left MCA occlusion in a 65-year-old man with acute onset of aphasia and right hemiparesis. The standard AP and lateral DSA images in the left panels show the region of interest (blue circle) used to compute the CFD parameter maps on the right that reveal decreasing velocity of retrograde blood flow, decreasing intraluminal pressures, and segmental changes in shear stress (from the left to right, respectively).
Theranostics of cerebral ischemia via precision imaging of the collaterome
The diverse spectrum of cerebral ischemia from the earliest manifestations to advanced disease may be characterized by imaging of the collaterome to transform our therapeutic strategies.10 Determining the optimal strategy to combat ischemia or augment collateral circulation requires fundamental understanding of hypoperfusion and the collaterome based on information that relates the individual to a much larger population. Big data and systematic analyses are necessary to develop normative standards, multimodal imaging atlases, and delineation of specific patterns to guide clinical management.21 Although widespread neurovascular imaging of healthy individuals is not feasible, the vast compendium of routinely acquired brain and vessel imaging across the population provides a platform for systematic analyses.21 In contrast to the traditional fragmentation or division of stroke and dementia, or alternatively the established designations of stroke subtypes without consideration of hypoperfusion and the collaterome, future approaches may leverage machine learning to recognize critical cerebral blood flow patterns.52 Serial imaging analyses will undoubtedly redefine the epidemiology and prognosis of ischemic brain disorders, including stroke and dementia. Importantly, the study of collaterals will move from isolated observations to methodical classification that may be used to match cases with the most appropriate hemodynamic and other vascular treatments. Such systematic imaging analyses are important to simultaneously identify modifying variables based on demographics, co-morbidities, and other relevant clinical data.21 The impact of hypoperfusion and specific patterns may be realized in many disorders from atrial fibrillation to small vessel disease and vascular cognitive impairment. The ultimate goal is to identify such features at the earliest epochs when prevention can realistically be implemented. Such theranostic strategies extend beyond simple diagnostics to allow for simultaneous therapeutic interventions based on imaging patterns of the collaterome.10 In acute ischemic stroke, a future theranostic strategy may entail real-time incorporation of diagnostic collateromic data provided by automated perfusion angiography or vesselography from each DSA run during endovascular therapy to directly guide next interventional or procedural steps in revascularization. In cases where limited collateral status, previously associated with impaired reperfusion, is noted, less aggressive or modest goals of revascularization may be indicated. Theranostics in intracranial atherosclerosis may include use of noninvasive CTA evaluation of fractional flow across a stenotic lesion with CFD to determine whether particular treatments such as angioplasty or medical strategies are tailored to a specific individual’s expected clinical course.53,54 As promoted by the BRAIN initiative and suggested by such examples of future theranostics, our understanding of fundamental pathophysiology with imaging technologies may directly lead to improvements in long-term neurological outcomes.55 Mapping the collaterome, however, does not solely rely on the development of novel technologies, as existing large-scale data from routinely available imaging around the world may be leveraged to define precision medicine in stroke and vascular dementia.2,36
Conclusions
Mapping the collaterome provides a trajectory to transform our current understanding of cerebral ischemia, at the transition of cerebrovascular health to the most advanced stages of vascular dementia. Precision cerebrovascular health may be guided by the influential role of collateral perfusion over time. Large-scale, systematic imaging analyses of the collaterome provide a platform for translational work on collateral circulation and hemodynamics in the brain and a theranostic framework with direct clinical implications.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (grant no. K24NS072272).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hinman JD, Rost NS, Leung TW, et al. Principles of precision medicine in stroke. J Neurol Neurosurg Psychiatr 2017; 88: 54–61. [DOI] [PubMed] [Google Scholar]
- 2.Liebeskind DS. Editorial commentary: Beyond the guidelines to expertise in precision stroke medicine. Trends Cardiovasc Med 2017; 27: 67–68. [DOI] [PubMed] [Google Scholar]
- 3.Smith EE, Saposnik G, Biessels GJ, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e44–e71. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 5.Liebeskind DS. Art of expertise in stroke telemedicine: imaging and the collaterome. Stroke 2015; 46: 610–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liebeskind DS. Imaging the collaterome: a stroke renaissance. Curr Opin Neurol 2015; 28: 1–3. [DOI] [PubMed] [Google Scholar]
- 7.Liebeskind DS, Feldmann E. Imaging of cerebrovascular disorders: precision medicine and the collaterome. Ann N Y Acad Sci 2016; 1366: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res 2017; 95(4): 943–972. [DOI] [PubMed] [Google Scholar]
- 9.de la Torre JC. Cerebral perfusion enhancing interventions: a new strategy for the prevention of Alzheimer dementia. Brain Pathol 2016; 26: 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res 2016; 7: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty A, de Wit NM, van der Flier WM, et al. The blood brain barrier in Alzheimer's disease. Vascul Pharmacol 2017; 89: 12–18. [DOI] [PubMed] [Google Scholar]
- 12.Donahue MJ, Hussey E, Rane S, et al. Vessel-encoded arterial spin labeling (VE-ASL) reveals elevated flow territory asymmetry in older adults with substandard verbal memory performance. J Magn Reson Imag 2014; 39: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskildsen SF, Gyldensted L, Nagenthiraja K, et al. Increased cortical capillary transit time heterogeneity in Alzheimer's disease: a DSC-MRI perfusion study. Neurobiol Aging 2016; 50: 107–118. [DOI] [PubMed] [Google Scholar]
- 14.Love S, Miners JS. Cerebral hypoperfusion and the energy deficit in Alzheimer's disease. Brain Pathol 2016; 26: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marnane M, Hsiung GY. Could better phenotyping small vessel disease provide new insights into Alzheimer disease and improve clinical trial outcomes? Curr Alzheimer Res 2016; 13: 750–763. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan M. Imaging small vessel disease: lesion topography, networks, and cognitive deficits investigated with MRI. Stroke 2010; 41: S154–S158. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Thrippleton MJ, Makin SD, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016; 36: 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Marco LY, Venneri A, Farkas E, et al. Vascular dysfunction in the pathogenesis of Alzheimer's disease – a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis 2015; 82: 593–606. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Sun Y, Lu Z, et al. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev 2017; 34: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebeskind DS, Kosinski AS, Lynn MJ, et al. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis. J Neuroimag 2015; 25: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebeskind DS. Crowdsourcing precision cerebrovascular health: imaging and cloud seeding a million brains initiative. Front Med (Lausanne) 2016; 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtman JW, Pfister H, Shavit N. The big data challenges of connectomics. Nat Neurosci 2014; 17: 1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011; 69: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab 2011; 31: 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faber JE, Moore SM, Lucitti JL, et al. Sex differences in the cerebral collateral circulation. Transl Stroke Res . Epub ahead of print 14 November 2016. DOI: 10.1007/s12975-016-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Wu B, Liu N, et al. Using standard first-pass perfusion computed tomographic data to evaluate collateral flow in acute ischemic stroke. Stroke 2015; 46: 961–967. [DOI] [PubMed] [Google Scholar]
- 27.Hattori Y, Enmi J, Iguchi S, et al. Gradual carotid artery stenosis in mice closely replicates hypoperfusive vascular dementia in humans. J Am Heart Assoc 2016; 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews PM, Hampshire A. Clinical concepts emerging from fMRI functional connectomics. Neuron 2016; 91: 511–528. [DOI] [PubMed] [Google Scholar]
- 29.Sporns O. Cerebral cartography and connectomics. Philos Trans R Soc Lond B Biol Sci 2015; 370(1668). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan SL, Sweet JG, Bishop N, et al. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke 2016; 47: 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucitti JL, Mackey JK, Morrison JC, et al. Formation of the collateral circulation is regulated by vascular endothelial growth factor-A and a disintegrin and metalloprotease family members 10 and 17. Circ Res 2012; 111: 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucitti JL, Sealock R, Buckley BK, et al. Variants of Rab GTPase-Effector Binding Protein-2 cause variation in the collateral circulation and severity of stroke. Stroke 2016; 47: 3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakar P, Zhang H, Chen D, et al. Genetic variation in retinal vascular patterning predicts variation in pial collateral extent and stroke severity. Angiogenesis 2015; 18: 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkhemer OA, Jansen IG, Beumer D, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke 2016; 47: 768–776. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Cancio E, Matheus MG, Romano JG, et al. Infarct patterns, collaterals and likely causative mechanisms of stroke in symptomatic intracranial atherosclerosis. Cerebrovasc Dis 2014; 37: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldmann E, Liebeskind DS. Developing precision stroke imaging. Front Neurol 2014; 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rostanski SK, Marshall RS. Precision medicine for ischemic stroke. JAMA Neurol 2016; 73: 773–774. [DOI] [PubMed] [Google Scholar]
- 38.Liebeskind DS. Big and bigger data in endovascular stroke therapy. Expert Rev Neurother 2015; 15: 335–337. [DOI] [PubMed] [Google Scholar]
- 39.Scalzo F, Liebeskind DS. Perfusion angiography in acute ischemic stroke. Comput Math Methods Med 2016; 2016: 2478324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams HP, Jr., Damasio HC, Putman SF, et al. Middle cerebral artery occlusion as a cause of isolated subcortical infarction. Stroke 1983; 14: 948–952. [DOI] [PubMed] [Google Scholar]
- 41.Alonso A, Arenas de Larriva AP. Atrial fibrillation, cognitive decline and dementia. Eur Cardiol 2016; 11: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 43.Choi BR, Kim DH, Back DB, et al. Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke 2016; 47: 542–547. [DOI] [PubMed] [Google Scholar]
- 44.Haller S, Zaharchuk G, Thomas DL, et al. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 2016; 281: 337–356. [DOI] [PubMed] [Google Scholar]
- 45.Roach BA, Donahue MJ, Davis LT, et al. Interrogating the functional correlates of collateralization in patients with intracranial stenosis using multimodal hemodynamic imaging. AJNR Am J Neuroradiol 2016; 37: 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H, Sun J, Ji X, et al. Correlation between the integrity of the Circle of Willis and the severity of initial noncardiac cerebral infarction and clinical prognosis. Medicine (Baltimore) 2016; 95: e2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu G, Yuan Q, Yang J, et al. The role of the circle of Willis in internal carotid artery stenosis and anatomical variations: a computational study based on a patient-specific three-dimensional model. Biomed Eng Online 2015; 14: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebeskind DS, Sansing LH. Willisian collateralization. Neurology 2004; 63: 344. [DOI] [PubMed] [Google Scholar]
- 49.Armitage GA, Todd KG, Shuaib A, et al. Laser speckle contrast imaging of collateral blood flow during acute ischemic stroke. J Cereb Blood Flow Metab 2010; 30: 1432–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stead LG, Bellolio MF, Gilmore RM, et al. Pharmacologic elevation of blood pressure for acute brain ischemia. Neurocrit Care 2008; 8: 259–261. [DOI] [PubMed] [Google Scholar]
- 51.Bergui M, Bradac GB. Progressive stroke, lacunae, and systemic blood pressure. Stroke 2002; 33: 2735–2736. [DOI] [PubMed] [Google Scholar]
- 52.Jia B, Scalzo F, Agbayani E, et al. Multimodal CT techniques for cerebrovascular and hemodynamic evaluation of ischemic stroke: occlusion, collaterals, and perfusion. Expert Rev Neurother 2016; 16: 515–525. [DOI] [PubMed] [Google Scholar]
- 53.Leng X, Scalzo F, Ip HL, et al. Computational fluid dynamics modeling of symptomatic intracranial atherosclerosis may predict risk of stroke recurrence. PLoS One 2014; 9: e97531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nam HS, Scalzo F, Leng X, et al. Hemodynamic impact of systolic blood pressure and hematocrit calculated by computational fluid dynamics in patients with intracranial atherosclerosis. J Neuroimaging 2016; 26: 331–338. [DOI] [PubMed] [Google Scholar]
- 55.Insel TR, Landis SC, Collins FS. Research priorities. The NIH BRAIN initiative. Science 2013; 340: 687–688. [DOI] [PMC free article] [PubMed] [Google Scholar]