Figure 1.
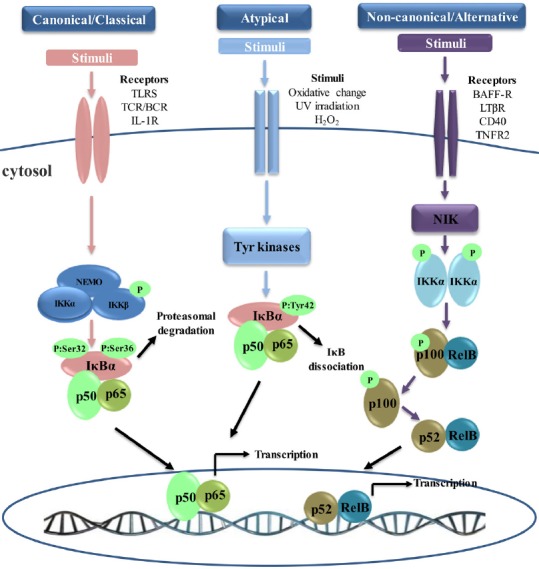
Nuclear factor κB (NF-κB) activation signaling pathways.
Canonical NF-κB pathway: the canonical pathway is inhibitor of κB (IκB) kinase (IKK)-dependent and IκB-dependent. Stimulation through receptors such as Toll-like receptors (TLRs) and T cell receptors (TCRs) leads to the activation of IKK complex, which phosphorylates IκBα and leads to its proteasomal degradation. Following the degradation of IκBα, heterodimer p65/p50 translocates to the nucleus and activates downstream gene transcription. Non-canonical NF-κB pathway: the non-canonical pathway is strictly dependent on IKKα, but independent of IKKβ, NF-κB essential modulator regulatory subunit (NEMO), and IκBα. Ligand-induced activation of receptors such as CD40 leads to the activation of NF-κB-inducing kinase (NIK). NIK specifically phosphorylates and activates IKKα homodimers. IKKα phosphorylation induces the polyubiquitination and proteasomal degradation of the NF-κB2 (p100) precursor to its active form, p52. Then, the newly formed heterodimer RelB/p52 translocates to the nucleus, resulting in the transcription of target genes. Atypical NF-κB Pathway: the atypical pathway is IKK-independent but IκBα dependen. This pathway is usually activated by the stimulation of the tyrosine kinase receptor or after oxidative challenge. After stimulation, IκBα is phosphorylated on Tyr 42 or on serine residues in the IκBα PEST domain. IκBα phosphorylation leads to the release of p65/p50, which translocates to the nucleus and transcribes the target genes. BCR: B cell receptors; IL-1R: interleukin-1 receptor; UV: ultraviolet; BAFF-R: B-cell activating factor receptor belonging to tumor necrosis factor family receptor; LTβR: lymphotoxin β-receptor; TNFR2: tumor necrosis factor receptor 2.
