Keywords: nerve regeneration, facial synkinesis, functional magnetic resonance imaging, neural plasticity, cortical representation, block design, facial movement, blinking, smiling, neural regeneration
Abstract
Facial synkinesis, a sequela of peripheral facial nerve palsy, is characterized by simultaneous involuntary facial movement during a voluntary desired one. Maladaptive cortical plasticity might be involved in the dysfunction of facial muscles. This cohort study investigated the cortical functional alterations in patients with unilateral facial synkinesis, using the task functional magnetic resonance imaging. Facial motor tasks, including blinking and smiling, were performed by 16 patients (aged 30.6 ± 4.5 years, 14 females/2 males) and 24 age- and sex-matched healthy controls (aged 29.1 ± 4.2 years, 19 females/5 males). Results demonstrated that activation in the cortico-facial motor representation area was lower during tasks in patients with facial synkinesis compared with healthy controls. Facial movements on either side performed by patients caused more intensive activation of the supplementary motor area on the contralateral side of the affected face, than those on the unaffected side. Our results revealed that there was cortical reorganization in the primary sensorimotor area and the supplementary motor area. This study was registered in Chinese Clinical Trial Registry (registration number: ChiCTR1800014630).
Introduction
A sequelae of severe peripheral facial nerve palsy is facial synkinesis, characterized by simultaneous involuntary facial movement during a voluntary desired one (Moran and Neely, 1996). The common synkinetic patterns, oral-ocular synkinesis and ocular-oral synkinesis, mainly involve the orbicularis oris muscles and the orbicularis oculi muscle, which are innervated by different branches of the facial nerve (Nakamura et al., 2003). The underlying pathological mechanism of facial synkinesis remains unanswered. Some researchers believe that the facial synkinesis following peripheral facial palsy is mainly due to the misguidance of regenerated axons, which have innervated the incorrect muscles (Montserrat and Benito, 1988). Paradoxically, the interval between facial nerve injury and the emergence of synkinesis in some cases is shorter than the theoretical period for any misdirected regeneration (May, 1983). Various animal studies demonstrated that the functional reorganization in the cortex following facial nerve injury could occur within hours after the onset of the lesion (Huntley, 1997). In addition, some investigators have shown changes of cortico-facial motor representations following facial nerve injury (Bitter et al., 2011; Klingner et al., 2011). Therefore, we considered that the central nervous system might be involved in dysfunction of facial muscles. Despite the well-established representation in the motor cortex for facial movements, we know of few studies that have investigated cortical functional alterations in patients with facial synkinesis. The current study aims to map the sensorimotor representation area of synkinetic movements by using block-designed functional magnetic resonance imaging (fMRI) with multiple facial actions.
Subjects and Methods
Subjects
A total of 16 patients with unilateral synkinesis following peripheral facial nerve palsy were randomly enrolled in this cohort study from the Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University, China. The control group was composed of 24 age- and sex-matched healthy subjects recruited publicly. For the patient group, the inclusion criteria consisted of: (1) a history of oral-ocular synkinesis for a period greater than 9 months, (2) absence of nerve transposition, and (3) the ability to follow instructions. Exclusion criteria for both groups were as follows: (1) confirmed or suspected history of cardiopulmonary failure, (2) psychiatric disorders; (3) concurrent peripheral neuropathy, and (4) contraindications to investigation by MRI. There were no significant differences in age or sex between the two groups. All patients showed a unilateral facial synkinesis (Table 1). The following fMRI study was approved by the Ethics Committee of the Shanghai Jiao Tong University (approval No. 2017-365-T267). Written informed consent was obtained from all volunteers and patients prior to the study. This study was registered in Chinese Clinical Trial Registry (registration number: ChiCTR1800014630). The flow chart for the procedures was shown in Figure 1.
Table 1.
Demographic information of facial synkinesis patients and healthy controls
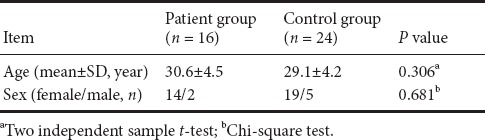
Figure 1.
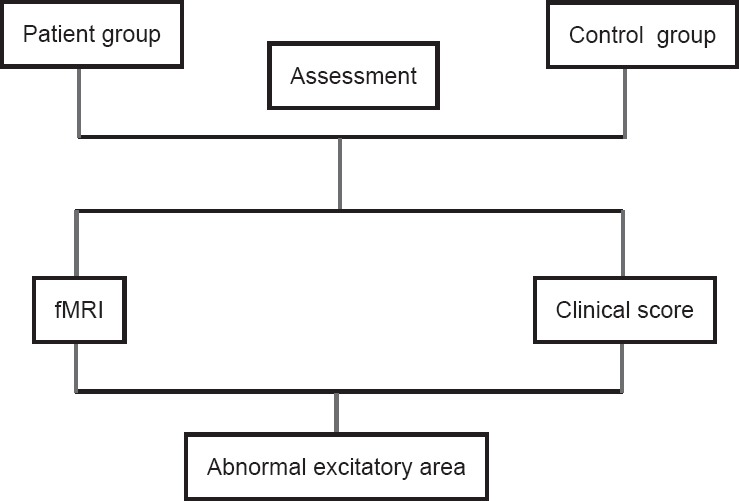
Flowchart of functional magnetic resonance imaging (fMRI) study in facial synkinesis patients and healthy controls.
Design and motor tasks
In the motor scans, participants were asked to execute four motor tasks: (1) Left eye blinking, (2) right eye blinking, (3) smiling on the left side, (4) smiling on the right side. The unilateral eye blinking was characterized by the rhythmic blinking of one eye, and smiling on the either side of the mouth was defined as unilateral contraction of the muscles innervated by a buccal branch. Patients were photographed for the above-mentioned tasks. During the scan, all subjects were instructed to perform repetitive movements rhythmically in response to visual signals. These were projected by a video projector onto a screen and reflected on a mirror attached to the head coil. Each action lasted for 30 seconds, alternating with another 30-second rest period. This was conducted three times. There were six blocks of fMRI scan for each task. The activation was calculated by comparison of the three scans of task and the three scans of rest. Before the beginning of the experiment, all subjects were trained on the tasks for approximately 20 minutes. The facial movements of each subject were recorded by camera.
Data acquisition
All imaging data were obtained with a 3.0 T GE Signa VH/I 3.0-T scanner (GE Healthcare, GE Asian Hub, Shanghai, China) equipped with a 32-channel head coil. The subject’s head was held between a pair of cushions, one on each side of the head, to minimize head movements. fMRI data were measured with an echo-planar imaging sequence (repetition time/echo time = 3000/35 ms, field of view = 240 mm × 240 mm, 43 axial slices, acquisition matrix = 64 × 64, voxel size = 3.4 mm × 3.4 mm × 3.2 mm, flip angle = 90°). Structural imaging data were acquired with a multiecho MPRAGE T1-weighted pulse sequence (repetition time/echo time = 1000/5 ms, TI = 1200 ms, flip angle = 200°, field of view = 240 mm × 240 mm, acquisition matrix = 256 × 256, sagittal acquisition, spatial resolution = 1 × 1 × 1 mm3, interslice space = 0 mm).
Data analysis
The image data were analyzed with the Statistical Parametric Mapping Program (SPM 8, Wellcome Institute for Imaging Neuroscience, London, UK) implemented in MATLAB (MathWorks, Natick, MA, USA). The EPI of each individual was realigned to the first image for each sequence separately for interscan movement artifacts. Their aligned functional images from all sessions were normalized by DARTEL to the Montreal Neurological Institute (MNI) standard brain for reporting MNI coordinates. Finally, the images were spatially smoothed using a 6-mm full width at half maximum Gaussian kernel. We made boxcar analysis with T-contrast for all sessions of every subject and retained only voxels with Z scores > 4.3946 for a P-value of 0.05 at voxel level with family-wise error correction for single-subject analysis. The group analysis for each session was performed using a one sample t-test. Voxels only with T scores > 2.8965 for a P-value of 0.05 at voxel level with false discovery rate correction were retained (Genovese et al., 2002). Before the analysis, the activation maps obtained during the movement task of the left side were flipped along the y-axis for assessing differences in the activation patterns between the motor tasks of the affected and unaffected side.
Results
Activation of brain regions during the smiling task in the healthy subjects and facial synkinesis patients
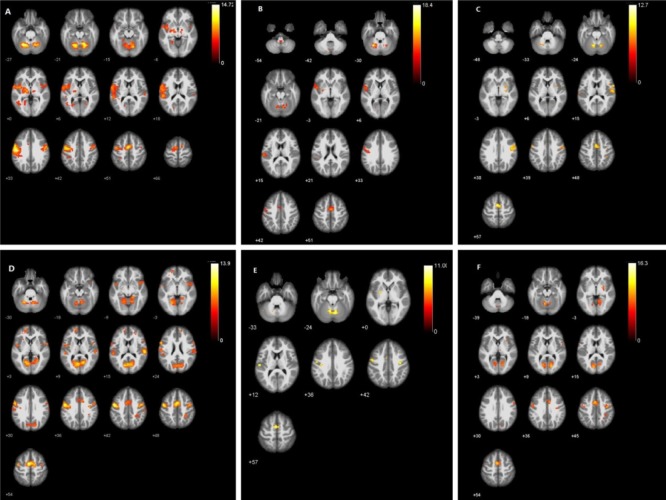
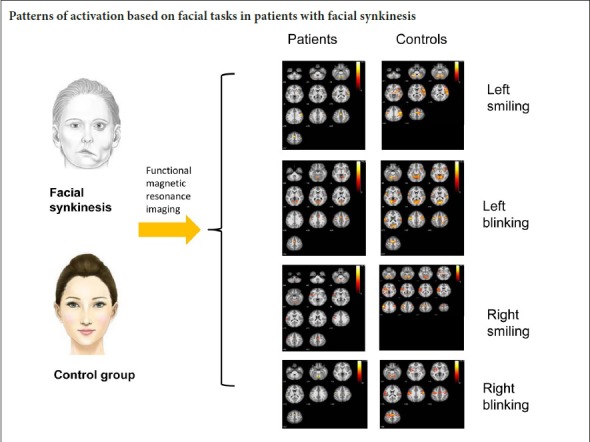
The control group showed significant activation of the contralateral primary sensorimotor cortex and minor activation of the ipsilateral primary sensorimotor cortex during the smiling task on either side. Activation also existed in the bilateral supplementary motor area (Figure 2A and Table 2).
Figure 2.
Activation maps of brain regions in healthy subjects and peripheral facial nerve palsy patients during facial movements.
(A) Activated brain regions in the control group during smiling. (B) Activated brain regions in the patient group on the affected side during smiling. (C) Activated brain regions in the patient group on the unaffected side during smiling. (D) Activated brain regions in the control group during blinking. (E) Activated brain regions in the patient group on the affected side during blinking. (F) Activated brain regions in the patient group on the unaffected side during blinking. The warm color bar is used to define the activation of the voxels. Red-yellow suggests a positive activation in the brain area. The numbers in the figures correspond to the slice numbers in the CH2 brain template. Control group: Healthy subjects; patient group: peripheral facial nerve palsy patients with unilateral synkinesis.
Table 2.
Brain regions activated by right-mouth movement in healthy subjects and right- or left-mouth movement in patients with facial synkinesis
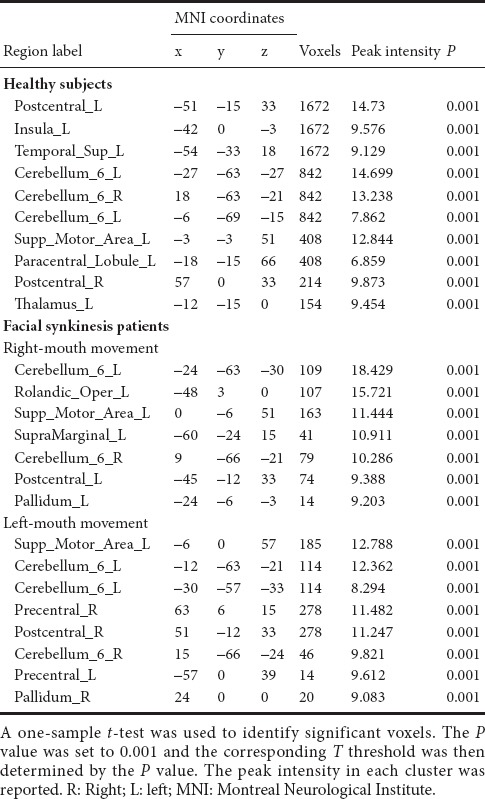
For the patient group, the smiling task on the affected side mainly activated the contralateral primary sensorimotor cortex and supplementary motor area (Figure 2B and Table 2). Compared with the former, the smiling task on the unaffected side (left side) caused more significant activation, especially in the primary sensorimotor cortex of the contralateral side (Figure 2C and Table 2).
Activation of brain regions during the blinking task in the healthy subjects and facial synkinesis patients
During the blinking task movement, the primary sensorimotor cortex and the supplementary motor area were bilaterally activated (Figure 2D and Table 3).
Table 3.
Brain regions activated by right-eyelid movement in controls and right- or left-eyelid movement in patients with facial synkinesis
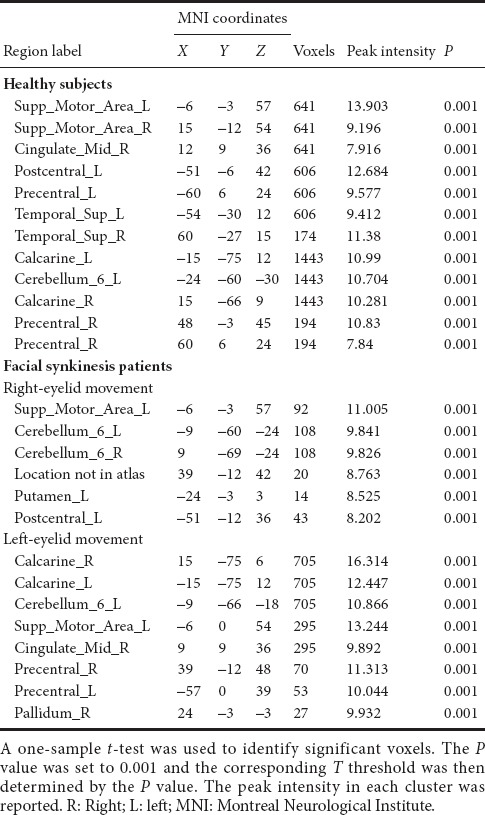
When performing the blinking task on the affected side (right side), patients showed activation of the contralateral primary sensorimotor cortex (Figure 2E and Table 3). Conversely, activation was found bilaterally in the primary sensorimotor cortices during the blinking task on the unaffected (left) side (Figure 2F and Table 3).
It is noteworthy that, for patients, facial movements on either side caused more intensive activity in the supplementary motor area on the unaffected side, than those on the affected side. Nevertheless, the activation during movements on the unaffected side of the patient group was lower than those in the control group.
Discussion
Facial synkinesis is one of the most unpleasant sequelae of peripheral facial nerve palsy. The efficacy of traditional surgical therapy, including high selective muscle resection and selective neurolysis, has not been satisfactory due to the serious complications or high recurrence rate (Husseman and Mehta, 2008). Despite the effects of botulinum toxin treatment to relieve the symptoms (Laskawi, 2008), facial synkinesis is extremely difficult to deal. The lack of clarity of the pathophysiological mechanism has been the main obstacle in contributing to the poor clinical outcomes. It is generally known that the injury and regeneration of peripheral nerve might result in plastic changes (Navarro et al., 2007), which can occur at multiple levels of the nervous system. Severe facial nerve damage results in synaptic reorganization in the facial nucleus, motoneuronal hyperexcitability and changes in the peripheral nerve activity (Oge et al., 2005). Investigations are necessary not only of the subcortical levels, including the peripheral nerves and brain stem, but also of the maladaptation of cortical reorganization. The present study provides an insight into the association between the clinical symptoms of facial synkinesis and the subserving activity pattern in the central nervous system. This is the first investigation that has attempted to map cortico-facial motor representations in facial synkinesis using fMRI.
Our results showed that in both the primary sensorimotor cortex and the premotor cortex, there are two distinct functional clusters devoted to the control of facial motor actions. This is basically consistent with a previous study (Morecraft et al., 2004), which indicates that the cortical network subserving facial motor function mainly consists of the primary motor cortex, the ventral lateral premotor cortex and the supplementary motor cortex. We also noticed activation in postcentral gyrus, even though the somatosensory afferents of the face are mediated by the trigeminal nerve and facial muscles have some internal sensory receptors. Bitter et al. (2011) have suggested that changes in representation of the face should be present only in the motor cortex, but not in the somatosensory cortex. However, animal studies have found that corticospinal neurons are present in the primary sensory cortex, which was verified by retrograde trans-synaptic tracing labeling (Rathelot and Strick, 2006). Another explanation is that motor cortex activity can drive changes in the network state of the somatosensory cortex through a cortico-cortical feedback pathway (Zagha et al., 2013). Some of our findings might indicate the close connection between the motor and somatosensory cortices in active motor paradigms even when only one system is involved.
In the control group, all facial representations seem to be bilaterally activated with a contralateral prevalence during unilateral movements. The areas activated by smiling (unilateral contraction of the risorius and zygomaticus) are mainly restricted in the contralateral motor cortex with minimal involvement of ipsilateral motor cortex. However, the cortical motor representation of blinking (eyelid movements) is symmetrical, with a slight predominance of contralateral activation. These results by and large confirmed the traditional viewpoint that the innervation of the face is bilaterally controlled for the upper part and mainly contralaterally controlled for the lower part (Cattaneo and Pavesi, 2014; Morecraft et al., 2004). The patient group, however, showed no comparable activation in the ipsilateral primary motor cortex during eyelid movements on the affected side. When they performed the same task contralaterally, the activation patterns were similar to those of the controls, i.e., there was bilateral activation of the primary motor cortex. One explanation could be that the ipsilateral cortical facial areas are spared, preventing hemisphere injury from an adverse impact on upper facial motor function. For eyelid movement, damage to the motor output site is more likely to cause eyelid dyskinesia than injury to the contralateral cortex.
Facial movements on either side performed in the patient group caused more intensive activation of the supplementary motor area on the contralateral side of the affected face, than those on the unaffected side. Nevertheless, in the control group, the supplementary motor areas bilaterally were markedly activated with contralateral prevalence during either facial task. A previous study revealed that more supplementary motor area neurons contributed to contralateral movements than to ipsilateral movements (Tanji et al., 1988). Accordingly, it is supposed that unilateral facial synkinesis was responsible for the above activation patterns in the supplementary motor area. In the brain, the supplementary motor area is one of vital areas that contribute to motor planning and modulates complex movement impulses transferred from the primary motor cortex to the motor nuclei within the brainstem. It also coordinates voluntary movements as well as involuntary movements (Nachev et al., 2008). A recent fMRI study demonstrated that the supplementary motor area is involved in the linkage of movements in different limbs, suggesting that even involuntary coordination of movement invokes a distributed network coordinated by the supplementary motor area (Salardini et al., 2012). It has been argued that supplementary motor area activity is evoked by the urge to move rather than the movement per se (Fried et al., 1991). Additionally, the increased activity of the supplementary motor area could be associated with the suppression of involuntary movements during the execution of self-paced movements (Finis et al., 2013). This could explain how the control of inappropriate movements, through increased awareness of imminent facial expressions, leads to the increased activity of the supplementary motor area.
Compared with healthy controls, patients with facial synkinesis demonstrated weak motor cortex activation during all tasks. We postulated that the maladaptive process is due to the denervation of facial muscles and the taking over of the functions of neighboring cortical areas after facial nerve injury. As described in a previous study, using transcranial magnetic stimulation and positron emission tomography, a shift of neighboring cortical areas replaced the deafferented area in severe facial palsy (Rijntjes et al., 1997). Remarkably for our patients, their activated voxel number and the average signal intensity in movements on the unaffected side were lower than those in controls, despite being still higher than those in affected counterparts. We presume that distinguishing of activation in different cerebral hemispheres was associated with the frequency of using facial muscles. Patients usually avoided the use of facial expressions, even on their unaffected side, to maintain the symmetry of facial movements.
The limitations of this study are as follows: The sample size is relatively small, and there is no detailed classification of the patient group. It lacks a comparison to a control group with unilateral complete facial paralysis.
In conclusion, our fMRI study reveals the patterns of activation based on facial tasks in patients with facial synkinesis. The recognition of multiple cortico-facial motor representations provides the opportunity to study the functional role of cortex neuroplasticity in facial synkinesis and how it impacts the control of facial movements. Physiotherapy modalities, including electromyogram biofeedback through neuromuscular re-education, have been found to be effective for facial synkinesis (Pourmomeny et al., 2014). This study explores cortical functional reorganization linked to facial synkinesis, and provides a theoretical basis for optimizing innovative medical therapies.
Acknowledgments
We are grateful to Jun-Tao Feng, the doctor of the Department of Hand Surgery from Huashan Hospital, China for his dedicated work in this study.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This study was supported by the Youth Researcher Foundation of Shanghai Municipal Commission of Health and Family Planning, No. 20144Y0095. The funder did not participate in data collection and analysis, paper writing or submission.
Institutional review board statement: This study was approved by the Ethics Committee of the Shanghai Jiao Tong University of China (approval number: 2017-365-T267). The study followed international and national regulations in accordance with the Declaration of Helsinki. The study was registered in Chinese Clinical Trial Registry (registration number: ChiCTR1800014630).
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study werereviewed by the biostatistician of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Muhammad Umer Nisar, University of Pittsburgh, USA.
Funding: This study was supported by the Youth Researcher Foundation of Shanghai Municipal Commission of Health and Family Planning, No. 20144Y0095.
(Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Bitter T, Sorger B, Hesselmann V, Krug B, Lackner K, Guntinas-Lichius O. Cortical representation sites of mimic movements after facial nerve reconstruction: a functional magnetic resonance imaging study. Laryngoscope. 2011;121:699–706. doi: 10.1002/lary.21399. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Pavesi G. The facial motor system. Neurosci Biobehav Rev. 2014;38:135–159. doi: 10.1016/j.neubiorev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Finis J, Enticott PG, Pollok B, Munchau A, Schnitzler A, Fitzgerald PB. Repetitive transcranial magnetic stimulation of the supplementary motor area induces echophenomena. Cortex. 2013;49:1978–1982. doi: 10.1016/j.cortex.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Huntley GW. Correlation between patterns of horizontal connectivity and the extend of short-term representational plasticity in rat motor cortex. Cereb Cortex. 1997;7:143–156. doi: 10.1093/cercor/7.2.143. [DOI] [PubMed] [Google Scholar]
- Husseman J, Mehta RP. Management of synkinesis. Facial Plast Surg. 2008;24:242–249. doi: 10.1055/s-2008-1075840. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Volk GF, Maertin A, Brodoehl S, Burmeister HP, Guntinas-Lichius O, Witte OW. Cortical reorganization in Bell’s palsy. Restor Neurol Neurosci. 2011;29:203–214. doi: 10.3233/RNN-2011-0592. [DOI] [PubMed] [Google Scholar]
- Laskawi R. The use of botulinum toxin in head and face medicine: an interdisciplinary field. Head Face Med. 2008;4:5. doi: 10.1186/1746-160X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. The facial nerve. Am J Otol. 1983;4:269. [PubMed] [Google Scholar]
- Montserrat L, Benito M. Facial synkinesis and aberrant regeneration of facial nerve. Adv Neurol. 1988;49:211–224. [PubMed] [Google Scholar]
- Moran CJ, Neely JG. Patterns of facial nerve synkinesis. Laryngoscope. 1996;106:1491–1496. doi: 10.1097/00005537-199612000-00009. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Toda N, Sakamaki K, Kashima K, Takeda N. Biofeedback rehabilitation for prevention of synkinesis after facial palsy. Otolaryngol Head Neck Surg. 2003;128:539–543. doi: 10.1016/S0194-59980223254-4. [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivó M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Oge AE, Yayla V, Demir GA, Eraksoy M. Excitability of facial nucleus and related brain-stem reflexes in hemifacial spasm, post-facial palsy synkinesis and facial myokymia. Clin Neurophysiol. 2005;116:1542–1554. doi: 10.1016/j.clinph.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Pourmomeny AA, Zadmehre H, Mirshamsi M, Mahmodi Z. Prevention of synkinesis by biofeedback therapy: a randomized clinical trial. Otol Neurotol. 2014;35:739–742. doi: 10.1097/MAO.0000000000000217. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci U S A. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijntjes M, Tegenthoff M, Liepert J, Leonhardt G, Kotterba S, Muller S, Kiebel S, Malin JP, Diener HC, Weiller C. Cortical reorganization in patients with facial palsy. Ann Neurol. 1997;41:621–630. doi: 10.1002/ana.410410511. [DOI] [PubMed] [Google Scholar]
- Salardini A, Narayanan NS, Arora J, Constable T, Jabbari B. Ipsilateral synkinesia involves the supplementary motor area. Neurosci Lett. 2012;523:135–138. doi: 10.1016/j.neulet.2012.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol. 1988;60:325–343. doi: 10.1152/jn.1988.60.1.325. [DOI] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]