Fig. 3.6.
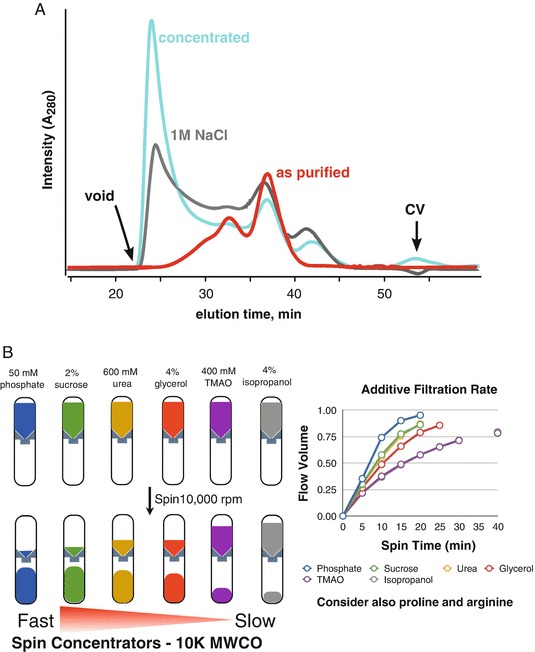
Sample instability during concentrating. Protein aggregation during concentrating is a routinely encountered problem and leads to exceedingly long concentrating times when using a spin-concentrator. The issue may not be relevant for most biochemistry experiments, however, it is a critical problem for SAXS. The WRN exonuclease domain was purified and concentrated in 20 mM Tris (7.6), 200 mM NaCl, and 5 mM beta-mercaptoethanol. (a) The protein was subjected to SEC analysis using a Superdex 200 PC 3.2 column in the same buffer (red trace). Upon concentrating the protein, the injected sample produced a significant UV signal in the void volume (gray trace). Concentrating the protein in buffer with 1 M NaCl increased the aggregation peak (cyan trace). (b) Additive screen using 10 K MWCO spin-concentrators. Various additives were added to the buffer and used to dilute the protein. For each additive, the filtration rate (volume of material that flowed through during centrifugation) was recorded at 5 min intervals. Samples contained phosphate (blue) and sucrose (green) had the fastest flow rates (Figure adapted from Kevin Dyer, Advanced Light Source, SIBYLS beamline, Berkeley, CA)
