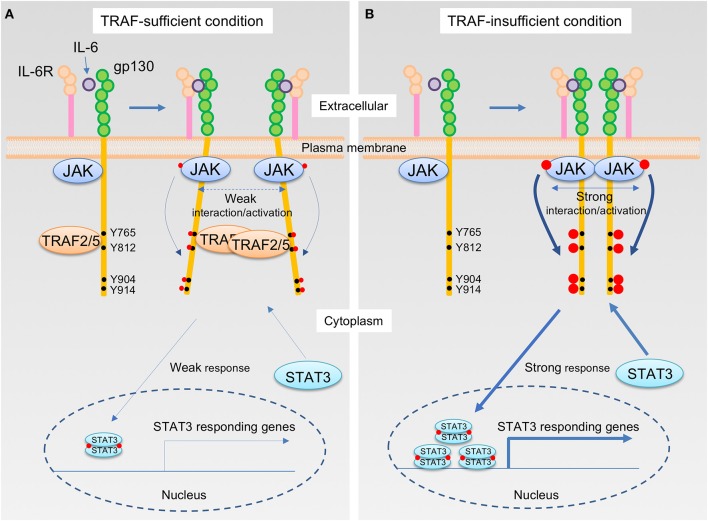
Figure 2.
The IL-6 receptor signaling pathway that is regulated by TRAF2 and TRAF5. (A,B) Upon interaction of IL-6 with the IL-6R, the complex of IL-6 and IL-6R next binds to the IL-6 receptor common chain gp130, which leads to dimerization of gp130. Janus kinase (JAK) is constitutively bound to the intracellular domains of gp130, and thus this event brings JAKs into close proximity, inducing transphosphorylation of each JAK on a tyrosine residue, indicated in red circle, that stimulates kinase activity of JAKs. The activated JAKs then phosphorylate the cytoplasmic tail of gp130 on specific tyrosine residues, generating binding sites for signal transducer and activator of transcription (STAT) including STAT3. Recruitment of a STAT3 to the phosphorylated gp130 brings the STAT3 close to the activated JAK, which then the activated JAK phosphorylates a tyrosine residue of the STAT3. Phosphorylated STAT3 molecules form a dimer, and STAT3 dimers translocate to the nucleus, then induce the gene transcription involved in TH17 differentiation, including RAR-related orphan receptor-γt (RORγt) and IL-17. TRAF2 and TRAF5 constitutively bind to a cytoplasmic region of the gp130, which includes an amino acid sequence 774VFSRSESTQPLLDSEERPEDLQLVD798 and locates between first two out of four distal phosphorylated tyrosine motifs in gp130, Y765, Y812, Y904, and Y914, that are recognized by STAT3. For this reason, JAK interactions are interrupted by the presence of TRAF2 and/or TRAF5, and this event causes a weaker interaction/activation of JAKs and subsequent attenuated responses in the IL-6 receptor signaling pathway (A). On the other hand, in the absence of TRAF2 and/or TRAF5, a stronger association of JAKs facilitates an augmented JAK activation that leads to the enhanced STAT3 responses in the IL-6 receptor signaling pathway (B).