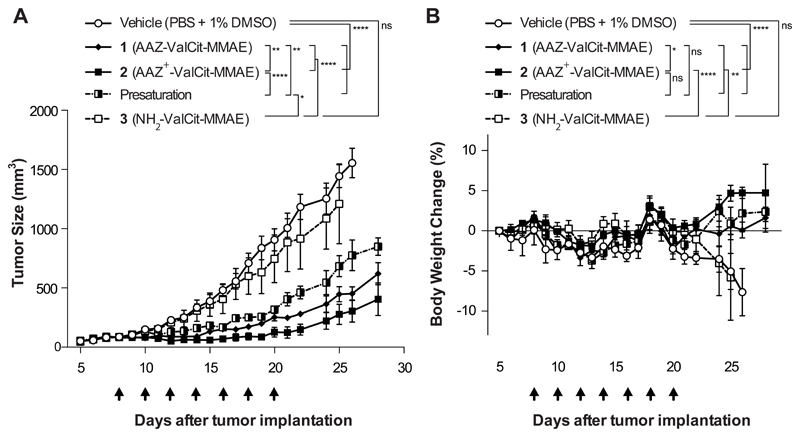
Figure 2. Comparison of the In vivo efficacy of AAZ-ValCit-MMAE and AAZ+-ValCit-MMAE (compounds 1 and 2) in BALB/c nu/nu mice bearing subcutaneous SKRC-52 renal cell carcinomas.
Cytotoxic derivative 3 devoid of the acetazolamide moiety was used as negative control. All the compounds were injected intravenously at the dose of 250 nmol/Kg per administration. The “presaturation” group was treated with a 50-fold dose of AAZ+ ligand (1.25 μmol/Kg; compound 4) directly followed by an administration of compound 2 (250 nmol/Kg). Graph (A) compares the therapeutic activity of the different treatment. Data points represent mean tumor volume ± SEM, n = 4 per group. SMDC 2 based on the affinity matured AAZ+ ligand exhibited a superior antitumor activity when compared with SMDC 1, based on the non-matured AAZ targeting moiety. The therapeutic efficacy of SMDC 2 was reduced significantly by the presaturation with an excess of free AAZ+. In graph (B) percentage changes of body weight during the experiment are represented. **** indicates p<0.0001; ** indicates p<0.01; * indicates p<0.05; ns indicates p>0.05 (2-way ANOVA test, followed by Bonferroni post-test).