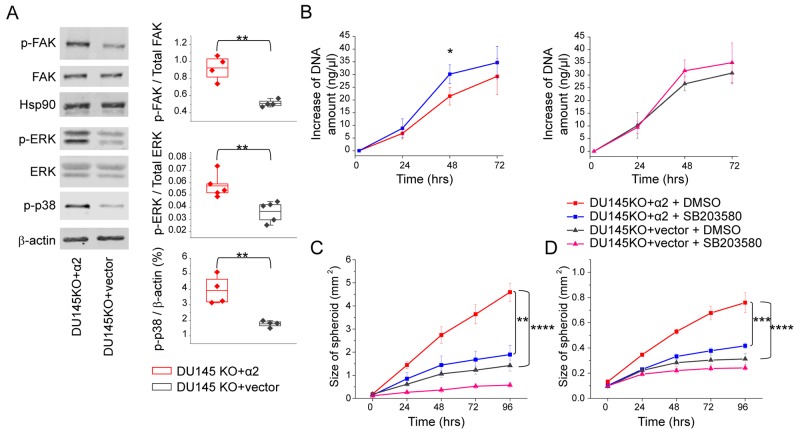
Figure 4. α2β1 integrin suppresses cell growth and promotes migration and invasion by increasing the phosphorylation of p38 MAPK.
(A) Representative western blot indicating that α2 integrin expression on DU145 prostate cancer cells increased phosphorylation of FAK, ERK and p38 MAPK proteins when cells were plated on collagen I coated surface. The quantification of the phosphorylated FAK/ total FAK and phosphorylated ERK/ total ERK is shown; phosphorylation of p38 MAPK is shown as % of β-actin (a loading control). Box plot shows data from 4 or 5 independent experiments (dots) and the mean from all experiments ±SEM. ** = P < 0.01. Student’s t-test. (B) p38 MAPK inhibitor SB203580 treatment (10 μg/ml) increased proliferation of DU145 cells. Proliferation of DU145KO+α2 and DU145KO+vector cells was assessed based on the change in DNA amount in 3D spheroids. Mean (n = 3) ±SEM. * = P < 0.05. Student’s t test. (C) Inhibition of p38 MAPK with SB203580 (10μg/ml) results significantly decreased migration of DU145KO+α2 cells on collagen I. Mean (n = 3) ±SEM. ** = P < 0.01, *** = P < 0.001. One way ANOVA and Tukey HSD post hoc test. (D) Invasion capability of DU145KO+α2 cells into collagen gel decreased significantly when cells were treated with p38 MAPK inhibitor SB203580 (10μg/ml). Mean (n = 3) ±SEM. *** = P < 0.001. One way ANOVA and Tukey HSD post hoc test.