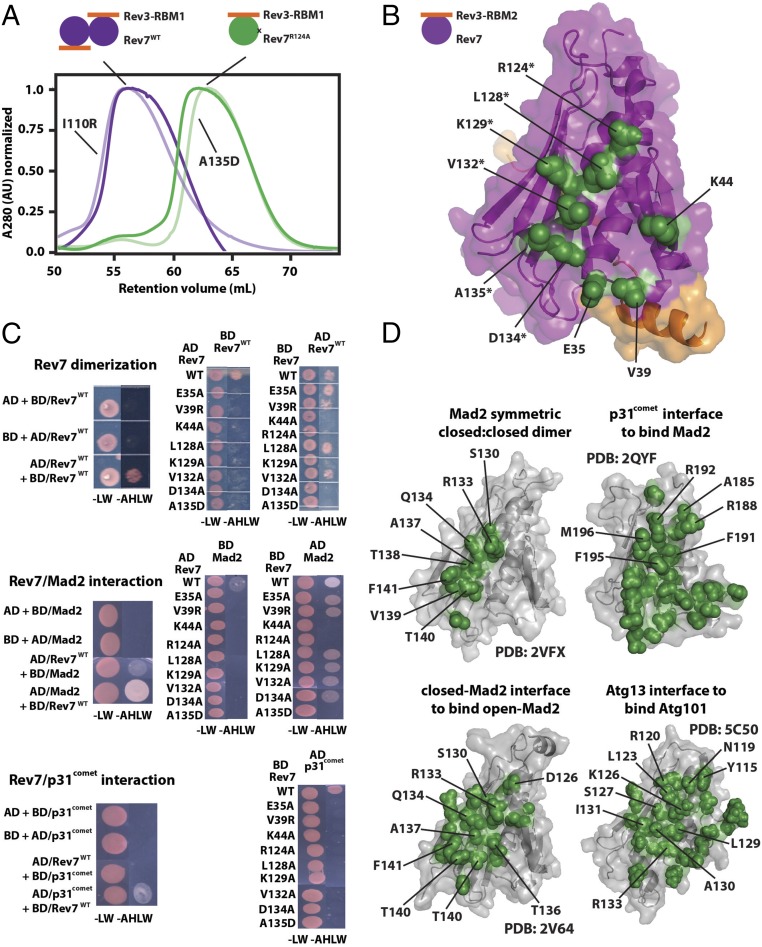
Fig. 2.
Mutational mapping of the Rev7 homo- and heterodimerization interface. (A) An example of gel filtration profiles of the monomeric Rev7R124A/Rev3–RBM1 (green) and dimeric Rev7WT/Rev3–RBM1 (purple) complexes; gel filtration profiles for additional Rev7 mutants which had no effect (I110R) or broke the dimer (A135D) are pictured in lighter shades. (B) Rev7 residues whose mutation abolishes dimerization (green) mapped on our structure of Rev7R124A/Rev3–RBM2, outlining the Rev7 dimerization interface. Residues on helix αC are marked with an asterisk. (C) Yeast two-hybrid studies of Rev7 homodimerization (Top) and its heterodimerization with Mad2 (Middle) or p31comet (Bottom): growth on −LW and −AHLW plates of the PJ69-4A strain of yeast transformed with fusions to the GAL4-BD or GAL4-AD as indicated. (D) Homo- and heterodimerization interface of other HORMA domains (47–49, 54). All structures appear in the same orientation as in C, and residues on helix αC are labeled.