Abstract
Context
Early life cortisol plays an important role in bone, muscle, and fat mobilization processes, which could influence body composition, affecting anthropometric indicators such as weight and height.
Objective
To explore the association between diurnal cortisol levels and growth indexes in children from 12 to 48 months of age.
Design
This study includes data from 404 children from the Programming Research in Obesity, Growth, Environment and Social Stressors Mexican birth cohort. Cortisol was measured in eight saliva samples collected at four time points during the day (from wakeup to bedtime), over 2 days, when the child was either 12, 18, or 24 months old. Total daytime cortisol levels were calculated by averaging the area under the curve (AUC) for the 2 days. Height and weight were measured from 12 to 48 months of age. Growth indexes were constructed according to z scores following World Health Organization standards: weight-for-age z score (Z-WFA), height/length-for-age z score, weight-for-height/length z score (Z-WFH), and body mass index–for-age z score (Z-BMIFA). Mixed models were used to analyze the association between cortisol AUC quartiles and growth indexes.
Results
Cortisol showed an inverted U-shaped association with the four growth indexes. Compared with the first quartile, all quartiles had a positive association with indexes that include weight, with the second quartile having the strongest association, resulting in an average change of β (95% CI) 0.38 (0.13–0.64) for Z-WFA, 0.36 (0.10–0.62) for Z-WFH, and 0.43 (0.17–0.69) for Z-BMIFA.
Conclusions
Results suggest that early life daytime cortisol levels, as a reflection of hypothalamic-pituitary-adrenal axis development, might influence growth in early infancy.
Cortisol plays an important role in metabolic processes. It could influence body composition, affecting anthropometric indicators such as weight and height in early childhood.
Cortisol plays an important role in peripheral tissues, with functions that include prioritization, use, and distribution of energy (1). Cortisol has a circadian rhythm that peaks in the morning and decreases during the day, reaching its lowest point during the night (1, 2). Normal daily cortisol concentrations are called basal levels, and when a person is under stress stimuli this hormone increases (1). Cortisol participates in physiological processes of bone, muscle, and fat mobilization, through glycogenolysis, gluconeogenesis, release of glucose to plasma, appetite stimuli, proteolysis of muscle, and inhibition of protein synthesis (3–7); hence, it is a crucial hormone during early child growth. However, cortisol’s role during growth has not been fully described.
Height and weight are influenced by nutrition, genetics, environment, and the hormonal system (8). Adequate growth during infancy enables proper physiological and social conditions in adult life (9). Deficient growth can lead to stunting and low school achievement and cognition (9, 10); by contrast, fast growth predicts health problems such as overweight, obesity, and chronic diseases (6, 11, 12). Children’s growth is monitored with charts, which are clinical tools that include four main indexes based on z scores: weight-for-age z score (Z-WFA), height/length-for-age z score (Z-HFA), and weight-for-height/length z score (Z-WFH). Additionally, the body mass index–for-age z score (Z-BMIFA) index is used for children >2 years of age (13).
Existing evidence shows that cortisol could influence children’s growth. A retrospective study has seen a relationship between higher adult cortisol levels and smaller anthropometric measurements in early childhood (6). In studies with children, findings are inconsistent. Whereas some studies report cortisol as positively associated with body mass index and weight in healthy 9-year-old children (5, 14), others have shown a negative correlation with body mass index, weight, and height in children and adolescents, one of them reporting an association with stunting in children (15–18). Studies that report no association with anthropometric measures (19, 20) had either a small sample size or children who were 5 years old. To our knowledge, there are insufficient studies on early life cortisol levels and growth. Therefore, the main aim of this study was to explore the association between basal daytime cortisol levels and growth in Mexican children from 12 to 48 months of age.
Methods
Study population
Our study population comes from the Programming Research in Obesity, Growth, Environment and Social Stressors Mexican birth cohort. From 2007 to 2011, we recruited healthy pregnant women at <20 weeks’ gestation from four family clinics at the Mexican Institute of Social Security. The inclusion criteria were being ≥18 years old, having access to a telephone, planned residence in Mexico City for the following 3 years, no use of steroids, no use of antiepileptic drugs, and not consuming alcohol on a daily basis (21). Participants had study visits every 6 months during the first 2 years of life and at 48 months of age. The inclusion criteria for this study were to have a cortisol measurement and at least one anthropometric measurement, which was met for 411 children. We excluded a child who was born without an arm and children who had a fever on the saliva collection day or had insufficient saliva samples (n = 6). A total of seven participants were excluded, and 404 children were included in our analysis. Saliva samples were collected at either 12 (n = 253), 18 (n = 100), or 24 (n = 51) months of age. Height and weight measurements were considered from the time of the saliva collection (cortisol measurement) until 48 months of age. Study protocols were approved by the institutional review boards of the National Institute of Public Health and the National Institute of Perinatology in Mexico and the Icahn School of Public Health and Harvard T. H. Chan School of Public Health in the United States. Written informed consent was obtained from all women before participation in the study.
Saliva collection and basal cortisol determination
At the 12-, 18-, or 24-month study visit, mothers were provided with an in-person explanation of the protocol, a diary, and the collection material, as well as instructions on how to collect samples. A total of eight saliva samples were collected over 2 days (four samples per day, from wakeup to bedtime): the first in the early morning (after the baby woke up and before the first meal) the second between 11:00 and 13:00 hours, the third between 15:00 and 17:00 hours, and the last sample 30 minutes after the last meal and before baby’s bedtime. Mothers were asked to register the exact time at which the samples were taken, information about sleeping and use of medicines in case of disease, and the exact hours of awakening, food times, naps, and sleep time. Mothers were asked to collect the samples ≤7 days apart and on typical days (not particularly stressful days), preferably when the child was not ill, taking medicine, or having an allergic reaction to food or an insect bite (22).
Saliva was collected on cotton braids, which the mother kept in the child’s mouth for 10 to 30 seconds, then drained into Salivette tubes (Sarstedt, Nümbrecht, Germany) with help of needleless syringes. Each sample was labeled with the exact time of collection and then refrigerated until the research team picked them up. Saliva samples were stored at −70°C in our research facility and sent to the laboratory of the Technical University of Dresden, Germany for analysis. Cortisol was calculated by duplicate with a commercial immunoassay technique that uses chemiluminescence, the Cortisol Saliva Luminescence Immunoassay (IBL Hamburg, Germany), with sensitivity of ~0.16 ng/mL and an intra-assay and interassay coefficient of variation <8% (22, 23).
Individual cortisol sample concentrations ±3 SD from the mean were excluded from our analysis. As an approximation of the daytime cortisol production we calculated the area under the curve (AUC), compounding repeated measurements and deriving a single measure via the trapezoidal rule (21, 23, 24). The AUC was determined by the sum of the three trapezoids formed below the curve. We plotted a cortisol curve for each participant and for each collection day, determined by the four measures in 1 day, and adjusted for the time since awakening. We then averaged the AUCs of the two collection days to increase stability. The slope of the cortisol AUC for each day indicates the velocity of cortisol decline during the day (23) and was calculated by subtracting the fourth and first cortisol measures. The slopes of the two days was also averaged. Any irregular or incomplete pattern was cross-checked with the diary information (22).
Anthropometry
Child’s length at 12 months of age was measured in a supine position with an infantometer. Weight at 12 months was measured with a Health-o-Meter pediatric strain gauge (Health-o-Meter, McCook, IL), making sure that the infant was wearing minimum clothes. For 18, 24, and 48 months of age, weight and height were measured with a Health-o-Meter physician strain gauge (Health-o-Meter) in a standing position. Birth weight was collected from the hospital’s birth discharge sheet. The anthropometric measurements were performed by staff using standardized procedures.
We identified three children with inconsistent length or height along the study period, probably because of a measurement error. After plotting them against the World Health Organization (WHO) 2006 charts and identifying discrepant values (e.g., height that decreased over time), we decided to treat these values as missing for the analysis.
Growth indexes
The z score values for Z-WFH, Z-WFA, Z-HFA, and Z-BMIFA, were calculated according to the WHO 2006 growth charts for each participant (13). For the analyses, growth indexes were used as a continuous variable. Indexes represent the distance of the value with respect to the reference mean; therefore, healthy children should report z scores close to the mean or a z score equal to zero.
Covariates
Based on previous research, the following covariates were considered for the analysis (6, 14, 17, 25): child’s sex, gestational age, birth weight, age in months at the time of the visit, and exact age in months at the time of cortisol measurement. Maternal information included height and education (total school years) measured at the second trimester of pregnancy and age at child’s birth.
We also explored feeding in the first 6 months of age as a proxy for energy intake and as an important covariate to explain variability in children’s growth data (26–28). Using breastfeeding information in the first and sixth months, we generated a variable with three categories: exclusively breastfed, exclusively formula-fed, and always mixed feeding and others (e.g., breastfeeding to formula, breastfeeding to mixed, formula to breastfeeding).
Statistical analysis
Participants with and without cortisol measurements were compared based on their sociodemographic characteristics. Comparison of categorical variables was performed via χ2 tests. For continuous variables we assessed normality and used t tests.
We used mixed models to analyze the average growth in children from 12 to 48 months. For each child, models could include up to four measures from the time of the cortisol measurement (12, 18, or 24 months) to 48 months of age. Weight and height/length were analyzed separately as dependent variables (adjusting for each other and covariates), and for our main analyses we used each growth index as a continuous variable adjusted for the covariates (separate models for each index). P < 0.05 was considered significant.
Using scatterplots with Lowess smoothing, we observed nonlinear associations between cortisol AUC and the growth indexes; therefore, quartiles were computed for the AUC. Models were adjusted for the average slope of the cortisol AUCs, also operationalized in quartiles. We tested for interactions between cortisol AUC and slope, cortisol AUC and child’s age, and cortisol AUC and child’s sex. All analyses were performed in Stata 13.0 (StataCorp LP, College Station, TX).
Results
Overall, the characteristics from the subjects with cortisol measurements did not differ from those with no cortisol measurements (Table 1). Just over a half of the children were boys. On average, mothers in our study were 27.23 ± 5.40 (mean ± SD) years old, had a height of 1.55 ± 0.05 m, and had finished high school. There was a higher prevalence of exclusive breastfeeding at 6 months of age in our study population than the reported Mexican prevalence (22% vs 14%) (29). Exclusive breastfeeding decreased 9 percentage points between the first and sixth months; use of formula increased 28 percentage points, and mixed feeding decreased 19 percentage points in the first 6 months of life. Ninety-one children had missing information on feeding. When we adjusted the models for this covariate, the effect estimates were larger; however, cortisol levels for children with feeding information were significantly higher than for those without the information (with feeding information, 6.4 ± 2.5 nmol/dL; missing feeding information, 5.7 ± 2.4 nmol/dL; P < 0.01). We therefore imputed the missing feeding information and reran the models. The results from the imputed models did not differ from the models excluding the feeding variable. Therefore, we did not include the feeding variable in our final models.
Table 1.
Characteristics of Participants With and Without Cortisol Measurement
| With Cortisol Measurement (n = 405) | With No Cortisol Measurement (n = 355) | P a | |
|---|---|---|---|
| Maternal information, mean ± SD | |||
| Height, m | 1.55 ± 0.05 | 1.55 ± 0.06 | 0.32 |
| Age, y | 27.23 ± 5.40 | 26.74 ± 5.51 | 0.22 |
| Education, y | 11.81 ± 2.80 | 11.84 ± 2.78 | 0.89 |
| Child’s information, mean ± SD | |||
| Female, N (%) | 187 (46.17%) | 173 (48.73%) | 0.48 |
| Gestational age, wkb | 38.40 ± 1.57 | 38.25 ± 1.90 | 0.22 |
| Birth length, cm | 49.70 ± 2.21 | 49.28 ± 2.30 | 0. 01 |
| Birth weight, kg | 3.11 ± 0.43 | 3.01 ± 0.46 | <0.01 |
| Breastfeeding at 1 mo, N (%)c | |||
| Exclusive | 108 (31.03%) | 95 (33.45%) | 0.60 |
| Use of formula | 46 (13.22%) | 42 (14.79%) | |
| Mixed feeding | 194 (55.75%) | 147 (51.76%) | |
| Breastfeeding at 6 mo, N (%)d | |||
| Exclusive | 77 (21.94%) | 57 (20.21%) | 0.83 |
| Use of formula | 146 (41.60%) | 123 (43.62%) | |
| Mixed feeding | 128 (36.47%) | 102 (36.17%) | |
| Height, cm, mean ± SDe | |||
| 12 mo | 74.30 ± 2.65 | 74.06 ± 2.68 | 0.29 |
| 18 mo | 79.27 ± 2.68 | 79.18 ± 2.86 | 0.70 |
| 24 mo | 83.81 ± 3.24 | 83.82 ± 3.57 | 0.98 |
| 48 mo | 105.62 ± 5.46 | 105.85 ± 5.35 | 0.62 |
| Weight (kg), mean ± SDf | |||
| 12 mo | 9.22 ± 1.12 | 9.02 ± 1.05 | 0.03 |
| 18 mo | 10.63 ± 1.39 | 10.47 ± 1.19 | 0.16 |
| 24 mo | 11.90 ± 1.55 | 11.72 ± 1.45 | 0.19 |
| 48 mo | 17.78 ± 3.49 | 17.55 ± 2.91 | 0.40 |
χ2 test for categorical variables and t test for continuous variables.
By last menstrual period method.
With cortisol measurement, n = 348; with no cortisol measurement, n = 284.
With cortisol measurement, n = 351; with no cortisol measurement, n = 282.
With cortisol measurement: 12 mo, n = 358; 18 mo, n = 364; 24 mo, n = 357; 48 mo, n = 345; with no cortisol measurement: 12 mo, n = 229; 18 mo, n = 196; 24 mo, n = 190; 48 mo, n = 248.
With cortisol measurement: 12 mo, n = 357; 18 mo, n = 365; 24 mo, n = 359; 48 mo, n = 345; with no cortisol measurement: 12 mo, n = 229; 18 mo, n = 197; 24 mo, n = 190; 48 mo, n = 248.
Descriptive statistics of cortisol AUC, z scores, and nutritional status are presented in Table 2. The range for the cortisol AUC at 12 months was 0.55 to 14 nmol/L, at 18 months it was 0.25 to 11.14 nmol/L, and at 24 months it was 1.12 to 14.09 nmol/L. As expected, we could see a decrease in cortisol concentrations as age increased (30): average AUC at 12 months showed the highest value, which decreased by 0.77 nmol/L by 18 months of age and then by 0.5 nmol/L at 24 months of age. The cortisol AUC quartiles had a mean ± SD of first quartile = 3.14 ± 1.1 nmol/L, second quartile = 5.48 ± 0.46 nmol/L, third quartile = 7.04 ± 0.52 nmol/L, and fourth quartile = 9.37 ± 1.28 nmol/L. Slope quartiles progressively had means of −1.50 ± 0.36, −0.94 ± 0.10, −0.61 ± 0.09, and −0.19 ± 0.20. We did not find statistically significant differences between boys’ and girls’ AUCs or slopes. The mean z scores at all growth points were within the normal limits (±2 SD) (13).
Table 2.
WHO Growth Index z Scores and Cortisol AUC
| 12 Mo, Mean ± SD | 18 Mo, Mean ± SD | 24 Mo, Mean ± SD | 48 Mo, Mean ± SD | |
|---|---|---|---|---|
| Cortisol AUCa (nmol/L) | 6.59 ± 2.56 | 5.82 ± 2.19 | 5.32 ± 2.29 | — |
| AUC slopea | −0.84 ± 0.55 | −0.77 ± 0.48 | −0.76 ± 0.52 | — |
| Z-WFA | −0.20 ± 1.03 | −0.11 ± 1.05 | −0.07 ± 1.03 | −0.13 ± 1.13 |
| Z-HFA | −0.38 ± 1.03 | −0.90 ± 0.92 | −0.96 ± 1.01 | −0.54 ± 0.90 |
| Z-WFH | −0.03 ± 1.11 | 0.42 ± 1.12 | 0.56 ± 1.01 | 0.20 ± 1.04 |
| Z-BMIFA | 0.02 ± 1.12 | 0.60 ± 1.12 | 0.73 ± 1.02 | 0.29 ± 1.15 |
AUC of cortisol for each study visit: 12 mo, n = 254; 18 mo, n = 100; 24 mo, n = 51.
In separate adjusted mixed linear models for weight and height, we observed that the second, third, and fourth cortisol AUC quartiles were positively associated with weight, compared with the first quartile of cortisol (reference category), showing an average increase across time of 0.56 kg (P < 0.001), 0.17 kg, and 0.12 kg, respectively. Height did not show a significant association with cortisol AUC (Table 3).
Table 3.
Mixed Models for Weight and Height at 12, 18, 24, and 48 Months With Cortisol AUC Quartiles at 12, 18, or 24 Months of Age
| Weighta |
Length or Heightb |
|||
|---|---|---|---|---|
| AUC Quartile | β (95% CI) | P | β (95% CI) | P |
| 2 | 0.56 (0.26, 0.87) | <0.001 | −0.07 (−0.58, 0.44) | 0.77 |
| 3 | 0.17 (−0.15, 0.48) | 0.29 | −0.41 (−0.94, 0.11) | 0.12 |
| 4 | 0.12 (−0.21, 0.45) | 0.48 | −0.07 (−0.63, 0.48) | 0.79 |
n = 404. Cortisol was measured at 12, 18, or 24 mo, and this time point was used as the basal time point for growth indexes. Models were adjusted for slope quartiles, child’s sex, gestational age (wk), birth weight (kg), age (mo), exact age at the time of cortisol measurement (mo), and maternal height (cm), age (y), and education (y). Boldface type indicates statistical significance.
Model for weight was adjusted for length or height.
Model for length or height was adjusted for weight.
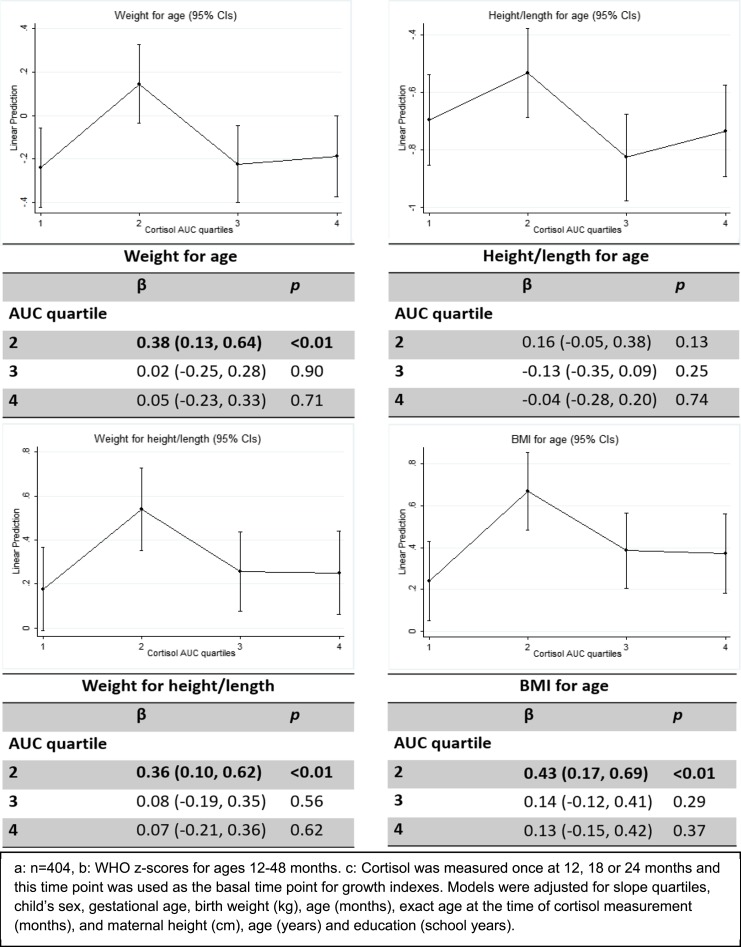
We computed and plotted the marginal effects for the adjusted predictor in the four models for the cortisol AUC quartiles and the different growth indexes (Fig. 1). A similar nonlinear relationship was found between cortisol AUC and the four growth indexes: an increase in the coefficient from the first to the second AUC quartile, followed by decreases to the third, and less so from the third to the fourth AUC quartile for Z-WFA and Z-BMIFA. Compared with the first cortisol AUC quartile, coefficients for the second cortisol quartile showed a positive association with Z-WFA, Z-WFH, and Z-BMIFA z scores of β (95% CI) 0.38 (0.13, 0.64), P < 0.01; 0.36 (0.10, 0.62), P < 0.01; and 0.43 (0.17, 0.69), P < 0.01, respectively. For the four indexes, the third and fourth quartiles showed a slight similar positive association. No significant results were found for the interactions tested (P > 0.05).
Figure 1.
Mixed models for WHO z scores at 12, 18, 24, and 48 mo for cortisol AUC quartiles at 12, 18, and 24 mo of age (n = 404). Cortisol was measured once at 12, 18, or 24 mo, and this time point was used as the basal time point for growth indexes.
Discussion
Children with either lower (first AUC quartile) or higher (third and fourth quartiles) cortisol levels showed smaller average growth z scores compared with those with moderate cortisol levels. For all growth indicators we saw a clear inverted U-shaped association. The growth indexes constructed from children’s weight had a significant association between the second AUC quartile and the first AUC quartile (reference): Z-WFA (0.38, P < 0.01), Z-WFH (0.36, P < 0.01), and Z-BMIFA (0.43, P < 0.01); however, this was not true for height, possibly indicating that cortisol is more closely related to weight. The first and fourth cortisol quartiles were associated with lower growth indexes across time for Z-WFA, Z-WFH, and Z-BMIFA, and the third and the fourth quartiles were associated with lower growth indexes for Z-HFA. Nonetheless, all coefficients were less than ±2 SD for the z scores, indicating that the average growth falls within normal values. Follow-up of these children’s growth will help us understand the relevance of the shape of the association found in our study.
Our results are consistent with the existing literature: a study of 152 healthy children, adolescents, and adults demonstrated a positive association of salivary cortisol levels in childhood with increasing body weight (5). Additionally, Francis et al. (14) reported a positive association between Z-BMIFA and higher cortisol concentration in 43 children between 5 and 9 years old, which was more evident in older children. The sample size for those studies was smaller than ours, and participants were >5 years old. A Brazilian study of 91 infants between 45 days and 36 months of age reported a negative association of weight, height, and body surface area (16). However, the study had important limitations: the small number of participants and the cortisol measurements (two measurements in one day, morning and afternoon) that might not be as representative of a daytime circadian rhythm. In our results, indicators that include weight for their conformation were closely related to the cortisol levels, and children with lower and higher basal cortisol concentrations showed lower weight gain during early childhood.
Cortisol has an important relationship with physiological bone processes; it participates in bone resorption and interferes in bone formation (3), affecting osteoblast and osteoclast function, promoting demineralization and a decrease in bone mass (31, 32). This relationship suggests a possible decrease in height or length, as seen in Cushing disease (3) and with glucocorticoid treatment (4). Nyberg et al. (33) reported higher cortisol concentrations and steeper slopes in stunted children 1.6 to 5.9 years of age in comparison with nonstunted children. Fernald and Grantham-McGregor compared 30 stunted and 24 nonstunted children between 8 and 10 years old and found higher salivary cortisol concentrations in the first group (17). Our findings for Z-HFA and the Z-WFH z score were not statistically significant; however, we saw that the highest cortisol levels (third and fourth quartile) had a negative association with respect to the lowest levels, indicating that cortisol could affect bone mass in children.
Biological cortisol processes are related with a person’s weight (1, 34), and a possible association between body mass and cortisol concentrations has been reported (5, 14). This association could be due to the use, distribution, and regulation of energy and the regulation of appetite, inhibiting leptin action (1, 14); however, hypothalamic-pituitary-adrenal axis function in obesity is still unknown (35). Kjölhede et al. (36) reported lower cortisol concentrations in overweight and obese children from 6 to 12 years old; however, the authors analyzed daytime cortisol concentrations separately (early morning, late morning, and evening) instead of an estimation of the total daytime cortisol. Another factor that could influence body mass is the proteolytic function of cortisol; this process increases the destruction of the muscle fibrils (4), therefore decreasing muscle mass and affecting body weight. Our findings are consistent with this evidence, but we saw that low or high cortisol concentrations could result in lower weight gain. We measured cortisol only at only one age time point for children; exploring this association with cortisol measures in older children undoubtedly will confirm our results.
Differences for weight and z score for Z-WFA at 12 months were statistically significant in our study participants (heavier by 100 g) compared with nonparticipants. Only children with saliva samples were included in this study. We could hypothesize that mothers who collected their child’s samples were slightly different from those who did not. We did not see any statistically significant differences between maternal characteristics. However, if mothers did not collect the sample because of, for example, higher daily stress, we could expect their children to have higher cortisol levels, and therefore our results might actually be underestimated.
Saliva samples show advantages in comparison with blood: they can be collected in newborns without causing stress, they are noninvasive, they can be taken by nonspecialized personnel, and only a small biological sample is needed (1, 5, 16, 37). Furthermore, cortisol has a passive transfusion from plasma to saliva (5), which is reflected in a high correlation with blood concentrations (38). Measuring daytime cortisol ideally captures its daily variation. Studies vary in the number of samples taken throughout the day, most ranging from two to five (24). Our study has the strength of having four samples per day (capturing the daytime cortisol rhythm) over 2 days, increasing precision for the basal cortisol measurement. In our study population, cortisol AUC and slope showed a decreasing tendency from 12 to 24 months of age, a finding that was similar to previously published evidence (30, 39). To our knowledge there are no reference values for infant and toddler daytime cortisol concentrations, and thus the values reported by our cohort could be used as reference for future research (22).
This is one of the first studies exploring the association between early life daytime cortisol and growth in children from 12 months to 4 years of age. Our results suggest that cortisol levels might be associated with children’s growth, specifically with indicators that contain weight for their conformation: Z-WFA, Z-WFH, and Z-BMIFA growth indexes.
Acknowledgments
We thank the Centro Médico ABC and the National Institute of Perinatology, México, for their support with this research. Authors from the National Institute of Public Health (INSP) are members of the Mexican Network for Children’s Environmental Health.
Financial Support: This work was supported by National Institute of Environmental Health Sciences Grants R01 ES013744, R01 ES014930, P42 ES016454, and P30 ES023515. It was also supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- WHO
World Health Organization
- Z-BMIFA
body mass index–for-age z score
- Z-HFA
height/length-for-age z score
- Z-WFA
weight-for-age z score
- Z-WFH
weight-for-height/length z score
References
- 1. Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34(2):271–292, vii. [DOI] [PubMed] [Google Scholar]
- 2. Tollenaar MS, Jansen J, Beijers R, Riksen-Walraven JM, de Weerth C. Cortisol in the first year of life: normative values and intra-individual variability. Early Hum Dev. 2010;86(1):13–16. [DOI] [PubMed] [Google Scholar]
- 3. Mazziotti G, Chiavistelli S, Giustina A. Pituitary diseases and bone. Endocrinol Metab Clin North Am. 2015;44(1):171–180. [DOI] [PubMed] [Google Scholar]
- 4. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18(10):1319–1328. [DOI] [PubMed] [Google Scholar]
- 5. Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, König A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–506. [DOI] [PubMed] [Google Scholar]
- 6. Power C, Li L, Hertzman C. Associations of early growth and adult adiposity with patterns of salivary cortisol in adulthood. J Clin Endocrinol Metab. 2006;91(11):4264–4270. [DOI] [PubMed] [Google Scholar]
- 7. Michels N, Sioen I, Braet C, Huybrechts I, Vanaelst B, Wolters M, De Henauw S. Relation between salivary cortisol as stress biomarker and dietary pattern in children. Psychoneuroendocrinology. 2013;38(9):1512–1520. [DOI] [PubMed] [Google Scholar]
- 8. Crespi B. The evolutionary biology of child health. Proc Biol Sci. 2011;278(1711):1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS; Maternal and Child Undernutrition Study Group . Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jolly R. Early childhood development: the global challenge. Lancet. 2007;369(9555):8–9. [DOI] [PubMed] [Google Scholar]
- 11. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–873. [DOI] [PubMed] [Google Scholar]
- 12. Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Singh H, Dahly DL, Bas I, Norris SA, Micklesfield L, Hallal P, Victora CG. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Onis M, Onyango AW; World Health Organization (WHO) . WHO child growth standards. Lancet. 2008;371(9608):204. [DOI] [PubMed] [Google Scholar]
- 14. Francis LA, Granger DA, Susman EJ. Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite. 2013;64:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Törnhage CJ, Alfvén G. Diurnal salivary cortisol concentration in school-aged children: increased morning cortisol concentration and total cortisol concentration negatively correlated to body mass index in children with recurrent abdominal pain of psychosomatic origin. J Pediatr Endocrinol Metab. 2006;19(6):843–854. [DOI] [PubMed] [Google Scholar]
- 16. Silva ML, Mallozi MC, Ferrari GF. Salivary cortisol to assess the hypothalamic-pituitary-adrenal axis in healthy children under 3 years old. J Pediatr (Rio J). 2007;83(2):121–126. [DOI] [PubMed] [Google Scholar]
- 17. Fernald LC, Grantham-McGregor SM. Stress response in school-age children who have been growth retarded since early childhood. Am J Clin Nutr. 1998;68(3):691–698. [DOI] [PubMed] [Google Scholar]
- 18. Dobrova-Krol NA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Cyr C, Juffer F. Physical growth delays and stress dysregulation in stunted and non-stunted Ukrainian institution-reared children. Infant Behav Dev. 2008;31(3):539–553. [DOI] [PubMed] [Google Scholar]
- 19. Koupil I, Mann V, Leon DA, Lundberg U, Byberg L, Vågerö D. Morning cortisol does not mediate the association of size at birth with blood pressure in children born from full-term pregnancies. Clin Endocrinol (Oxf). 2005;62(6):661–666. [DOI] [PubMed] [Google Scholar]
- 20. Netherton C. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29(2):125–140. [DOI] [PubMed] [Google Scholar]
- 21. Braun JM, Wright RJ, Just AC, Power MC, Tamayo Y Ortiz M, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health. 2014;13(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamayo y Ortiz M, Téllez-Rojo MM, Wright RJ, Coull BA, Wright RO. Longitudinal associations of age and prenatal lead exposure on cortisol secretion of 12–24 month-old infants from Mexico City. Environ Heal. 2016;15(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schreier HMC, Hsu H-H, Amarasiriwardena C, Coull BA, Schnaas L, Téllez-Rojo MM, Tamayo y Ortiz M, Wright RJ, Wright RO. Mercury and psychosocial stress exposure interact to predict maternal diurnal cortisol during pregnancy. Environ Health. 2015;14(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69(7):651–659. [DOI] [PubMed] [Google Scholar]
- 25. Ng PC. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F250–F254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate. 1998;74(2):94–105. [DOI] [PubMed] [Google Scholar]
- 27. Lok KYW, Chau PH, Fan HSL, Chan KM, Chan BH, Fung GPC, Tarrant M. Increase in weight in Low Birth weight and very low birth weight infants fed fortified breast milk versus formula milk: a retrospective cohort study. Nutrients. 2017;9(5):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scherbaum V, Srour ML. The role of breastfeeding in the prevention of childhood malnutrition. World Rev Nutr Diet. 2016;115:82–97. [DOI] [PubMed] [Google Scholar]
- 29. Instituto Nacional de Salud Pública Encuesta Nacional de Salud y Nutrición: Resultados Nacionales 2012. Cuernavaca, Morales, Mexico: Instituto Nacional de Salud Pública; 2012:200. [Google Scholar]
- 30. Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45(3):125–133. [DOI] [PubMed] [Google Scholar]
- 31. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu K, Henley D, Pennell C, Herbison CE, Mountain J, Lye S, Walsh JP. Associations between hypothalamic-pituitary-adrenal axis function and peak bone mass at 20 years of age in a birth cohort. Bone. 2016;85:37–44. [DOI] [PubMed] [Google Scholar]
- 33. Nyberg CH, Leonard WR, Tanner S, McDade T, Huanca T, Godoy RA; Taps Bolivia Study Team . Diurnal cortisol rhythms and child growth: exploring the life history consequences of HPA activation among the Tsimane’. Am J Hum Biol. 2012;24(6):730–738. [DOI] [PubMed] [Google Scholar]
- 34. Solano JM, Jacobson L. Glucocorticoids reverse leptin effects on food intake and body fat in mice without increasing NPY mRNA. Am J Physiol. 1999;277(4 Pt 1):E708–E716. [DOI] [PubMed] [Google Scholar]
- 35. Reynolds RM, Syddall HE, Walker BR, Wood PJ, Phillips DIW. Predicting cardiovascular risk factors from plasma cortisol measured during oral glucose tolerance tests. Metabolism. 2003;52(5):524–527. [DOI] [PubMed] [Google Scholar]
- 36. Kjölhede EA, Gustafsson PE, Gustafsson PA, Nelson N. Overweight and obese children have lower cortisol levels than normal weight children. Acta Paediatr. 2014;103(3):295–299. [DOI] [PubMed] [Google Scholar]
- 37. Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008;11(1):1–14. [DOI] [PubMed] [Google Scholar]
- 38. Gallagher P, Leitch MM, Massey AE, McAllister-Williams RH, Young AH. Assessing cortisol and dehydroepiandrosterone (DHEA) in saliva: effects of collection method. J Psychopharmacol. 2006;20(5):643–649. [DOI] [PubMed] [Google Scholar]
- 39. Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. [DOI] [PubMed] [Google Scholar]