Abstract
Context
It is unclear if effects of glucagon-like peptide-1 (GLP-1) and clinically available GLP-1 agonists on the heart occur at clinical doses in humans, possibly contributing to reduced cardiovascular disease risk.
Objective
To determine whether liraglutide, at clinical dosing, augments myocardial glucose uptake (MGU) alone or combined with insulin compared with insulin alone in metformin-treated type 2 diabetes mellitus (T2D).
Design
In a randomized clinical trial of patients with T2D treated with metformin plus oral agents or basal insulin, myocardial fuel use was compared after 3 months of treatment with insulin detemir, liraglutide, or combination detemir plus liraglutide added to background metformin.
Main Outcome Measures
Myocardial blood flow (MBF), fuel selection, and rates of fuel use were evaluated using positron emission tomography, powered to demonstrate large effects.
Results
MBF was greater in the insulin-treated groups [median (25th, 75th percentile): detemir, 0.64 mL/g/min (0.50, 0.69); liraglutide, 0.52 mL/g/min (0.46, 0.58); detemir plus liraglutide, 0.75 mL/g/min (0.55, 0.77); P = 0.035 comparing three groups, P = 0.01 comparing detemir groups to liraglutide alone]. There were no evident differences among groups in MGU [detemir, 0.040 µmol/g/min (0.013, 0.049); liraglutide, 0.055 µmol/g/min (0.019, 0.105); detemir plus liraglutide, 0.037 µmol/g/min (0.009, 0.046); P = 0.68 comparing three groups]. There were no treatment-group differences in measures of myocardial fatty acid uptake or handling, and no differences in total oxidation rate.
Conclusion
These observations argue against large effects of GLP-1 agonists on myocardial fuel metabolism as mediators of beneficial treatment effects on myocardial function and ischemia protection.
In PET studies, liraglutide did not alter myocardial fuel selection in metformin-treated T2D. This argues against a myocardial metabolic effect in cardiovascular disease benefits of GLP-1 treatments.
Glucagon-like peptide 1 (GLP-1)–based treatments have been developed for the management of type 2 diabetes mellitus (T2D). Shortly after the discovery of GLP-1 and its effects via the pancreas to regulate glucose metabolism, GLP-1 was found to have extrapancreatic effects, including modulating myocardial metabolism and function (1). Specifically, GLP-1 exposure can acutely augment myocardial glucose uptake (MGU), and this effect has been postulated to contribute to cardiac protection under ischemia and to improvements in cardiac function (2). We and others have shown that these effects can be replicated in small and large animal models and in lean nondiabetic humans (3–7). Importantly, however, we have demonstrated an impairment in myocardial metabolic effects of GLP-1 agonism in humans with T2D and in obese, nondiabetic swine (3). This is potentially important because the clinical populations treated with GLP-1 agonists have obesity and/or T2D.
This impairment in GLP-1 metabolic responses in the heart is parallel to reports of impaired myocardial responses to insulin in obesity and in T2D (8). The genesis of myocardial insulin resistance is not fully explained, but it has been attributed in part to impairment of myocardial insulin signaling, leading to impaired insulin-stimulated glucose uptake (9), and in part to augmented fatty acid uptake by the heart (10), leading to impaired myocardial metabolic flexibility. GLP-1 appears to exert effects directly on the heart to drive MGU, via pathways that are independent of myocardial insulin signaling (3, 11). This suggests the possibility that GLP-1 and insulin actions on MGU could converge, and perhaps GLP-1 could help overcome myocardial insulin resistance (12).
The actions of current GLP-1 agonists on the myocardium in people with T2D have not been fully described. It is unknown whether the doses developed and approved for the management of glycemia exert metabolic actions on the heart, and it is unknown how those actions relate to the effects of insulin on heart metabolism. We hypothesized that a GLP-1 mimetic in combination with insulin would provide superior stimulation of MGU compared with either agent alone. Therefore, we undertook a randomized clinical trial using positron emission tomography (PET), powered to demonstrate large effects, to compare the effects of insulin detemir, liraglutide, or the two combined, on myocardial fuel metabolism in people with T2D.
Methods
Population
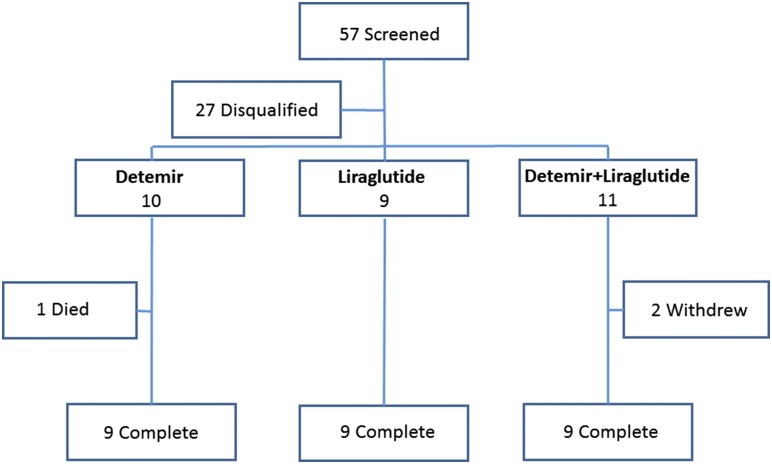
Volunteers were recruited by advertising. Inclusion criteria included T2D of at least 2 years’ duration stably treated with metformin ≥1500 mg/d, age 18 to 50 years, HbA1c range from 7.0% to 10.0%, and treatment with up to two oral antidiabetic agents and/or basal insulin. Key exclusion criteria included exposure within the past 6 months to a dipeptidyl peptidase-4 inhibitor, GLP-1 agonist, or diabetes agent in the thiazolidinedione class; known coronary artery disease or structural heart disease; known diabetic microvascular disease (ratio of urinary albumin to creatinine >30 mg/g, diagnosed retinopathy or neuropathy by self-report, or either of these detected on screening physical examination); and contraindications to radiation exposure with planned PET. The flow of participants through the study is presented in Fig. 1. All participants gave written informed consent for their participation in the study, and the study was conducted according to the Helsinki principles. This study was registered with ClinicalTrials.gov (identifier: NCT01232946).
Figure 1.
Flow of study participants. One participant in the detemir-only arm died of non-study-related causes during randomized treatment.
Treatment
Volunteers were randomly assigned in a fixed-block design to one of three treatments: insulin, liraglutide, or combination insulin and liraglutide. Antidiabetes agents other than metformin were discontinued, and study treatment was substituted in addition to ongoing metformin; if participants were taking submaximal metformin dosing, an attempt was made to increase this to 2000 mg/d before randomization. The long-acting insulin detemir was used, with dosing initiated at 5 to 10 units twice daily (depending on the current blood glucose readings) and titrated twice weekly to achieve fasting glucose values of <120 mg/dL (6.7 mmol/L) without values dropping below 80 mg/dL (4.4 mmol/L). Liraglutide dosing followed the product labeling, starting with 0.6 mg/d initially and increased weekly to 1.8 mg/d. Participants randomly assigned to combination therapy first took liraglutide and had insulin added when stable liraglutide dosing was achieved. PET measurements of myocardial metabolism were planned at the end of 12 weeks of study-assigned treatment.
Study measurements
Subjects were admitted to the Indiana University Clinical Translational Sciences Institute's Clinical Research Center before 7:00 am on the morning of the PET study, following an overnight fast (from 8:00 pm the day prior). Anthropometrics, blood pressure, and heart rate were measured in a standardized manner. Body composition was measured using dual-energy X-ray absorptiometry. An intravenous catheter was placed in the antecubital vein of one arm for infusion. A second catheter was placed in the contralateral arm for blood sampling.
Subjects treated with liraglutide received a subcutaneous injection of liraglutide 1.8 mg at 8:00 am on the morning of the PET study. Subjects treated with insulin took their stable morning insulin detemir dose also at 8:00 am the morning of the study. The PET measurement protocol began between 10:00 am and 11:00 am.
PET protocol
PET was used to measure cardiac perfusion and oxidative metabolism (via the radiotracer 11C-acetate), fatty acid uptake and oxidation (via the radiotracer 11C-palmitate), and glucose uptake (via the radiotracer 18F-deoxyglucose) under resting fasting conditions with hormone exposure. Blood samples for later measurement of circulating concentrations of glucose, insulin, and nonesterified fatty acids were taken at the beginning and end of the PET measurement sequence, with average values calculated to reflect exposure during the PET measurements.
Conventional data acquisition procedures were used to acquire and reconstruct the PET images. This included a transmission measurement to enable correction for attenuation by body mass. The image data were reconstructed using conventional, filtered, back-projection algorithms and a Hanning smoothing filter, which produces an image resolution of 1.0 cm full-width at half-maximum. This filter function was selected because it is consistent with the observed resolution degradation observed by the motion of the heart as determined with cardiac-gated imaging studies. Custom software was used to identify the entire left ventricle as a single volume of interest and then apply mathematical modeling to the resulting volume of interest. The left ventricle cavity, concurrently identified, served as an arterial blood pool from which the input function was derived.
After acetate infusion, serial, timed peripheral blood samples were taken (at minutes 0, 3, 6, 10, 15, 20, 25, and 30), immediately analyzed for circulating concentrations of labeled CO2, and later used as a correction for the input function (13, 14). Timed samples were taken at these same intervals after palmitate infusion and assayed for total and palmitate-specific radioactivity to determine metabolite corrections for this analyte’s input function. Tissue perfusion and total oxidative metabolism were quantified from the myocardial time-activity curve after 11C-acetate injection, using a well-validated, two-compartment model (13, 15). Myocardial blood flow (MBF) was measured directly from the acetate influx kinetic parameter, K1 (15, 16). Myocardial oxygen consumption (MVO2) was derived from the acetate efflux parameter k2 using the following equation: MVO2 = 135 × k2 − 0.96 (17). A three-compartment, three-free parameter model including uptake, oxidation, and esterification kinetics was used to derive rates of fatty acid use from the palmitate time-activity curves (18), with net rates of fatty acid oxidation and esterification calculated according to the method of Bergmann (19). MGU was quantified from the time-activity curve after 18FDG injection using a three-compartment model, according to the methods of Morita et al. (20), with a lumped constant of 1.0 (21, 22).
Sample size estimation and statistical approach
MGU was our primary end point for analysis; other PET-derived measures were co-secondary end points. We were specifically interested in whether the combined exposure produced myocardial metabolic rates greater than exposure to insulin alone. Our prespecified plan for statistical testing was constructed to first evaluate whether the three treatment groups were different with post hoc pairwise testing if indicated, and then to compare two liraglutide groups against insulin alone and two insulin groups against liraglutide alone. Post hoc analyses including multivariable analyses with relevant covariates were planned as exploratory analyses; due to unexpected group differences in some baseline factors, post hoc sensitivity analyses were performed to evaluate direct and interaction effects of these factors on the main three-group outcome. P < 0.05 was considered significant throughout analyses without adjustment for multiple testing.
Power estimates and sample-size calculations were based on the primary end point of MGU. Our prior work suggested that the mean ± SD MGU in T2D would be ∼0.026 ± 0.016 μmol/g/min, and studies by others suggested that diabetes therapies added to background treatments could induce 30% to 40% increases in MGU (i.e., effect sizes of ∼0.7 SD) (23, 24). We estimated that a sample size of nine participants per group would permit demonstrating between-group differences of ∼1.2 SD with α = 0.05 and β = 0.2 for MGU. Therefore, the study was powered to demonstrate large effects, if present. Working from this glucose uptake–related sample size, we estimated demonstrable between-group differences of ∼0.5 SD for MBF, ∼1.4 SD for total myocardial oxidation rates, and ∼0.6 SD for myocardial fatty acid oxidation (MFAO) rates.
The PET data for metabolite rates were right skewed and not adequately normalized by standard transformations (i.e., logarithmic, square root, inverse). Therefore, we applied nonparametric testing, using Kruskal-Wallis tests to compare across three treatment groups and Mann-Whitney U tests for pairwise testing. These skewed distributions were best represented in the figures by plotting median and 75th percentile values. Sensitivity analyses evaluating the potential effects of baseline group differences were performed using standard ANOVA with untransformed data excluding extreme outliers, to meet the assumptions of the method.
Results
The flow of participants through the study is presented in Fig. 1, and the characteristics of the participants who enrolled and completed the study are presented in Table 1. Two participants originally randomly assigned to receive combination insulin detemir plus liraglutide withdrew; one experienced intolerable adverse gastrointestinal effects and another was lost to follow-up without a known reason for withdrawal. One participant originally randomly assigned to the detemir arm died of a narcotic overdose after randomization.
Table 1.
Participant Characteristics
| Variable | Detemir | Liraglutide | Detemir + Liraglutide | P Valuea (ANOVA) |
|---|---|---|---|---|
| Race, black/white, no. | 3/6 | 3/6 | 7/2 | 0.090 |
| Sex, female/male, no. | 7/2 | 2/7 | 8/1 | 0.007 |
| Age, y | 52.1 ± 8.6 | 53.9 ± 5.3 | 49.2 ± 9.0 | 0.45 |
| Weight, kg | 94.8 ± 20.0 | 99.3 ± 17.9 | 88.7 ± 19.6 | 0.50 |
| Height, m | 1.64 ± 0.09 | 1.74 ± 0.06 | 1.61 ± 0.04 | 0.002 |
| BMI, kg/m2 | 35.1 ± 7.2 | 33.4 ± 6.8 | 34.2 ± 6.9 | 0.87 |
| Total cholesterol, mmol/L | 3.4 ± 1.4 | 3.8 ± 1.4 | 3.8 ± 0.8 | 0.77 |
| LDL cholesterol, mmol/L | 1.8 ± 1.4 | 2.2 ± 1.2 | 2.2 ± 0.9 | 0.76 |
| HDL cholesterol, mmol/L | 0.9 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.2 | 0.42 |
| Triglyceride, mmol/L | 1.9 ± 1.1 | 2.0 ± 1.0 | 1.5 ± 0.9 | 0.60 |
| Glucose, mmol/L | 6.5 ± 2.6 | 9.4 ± 3.2 | 9.3 ± 3.1 | 0.084 |
| HbA1c, randomization, % | 7.00 ± 0.79 | 7.59 ± 0.96 | 8.13 ± 0.99 | 0.060 |
| HbA1c, randomization, mmol/mol | 53 ± 6 | 60 ± 8 | 64 ± 9 | 0.060 |
Values are mean ± SD unless otherwise indicated.
The P value represents testing for a difference among the three groups by analysis of variance.
The most notable feature of the baseline characteristics was the unbalanced sex distribution across treatment groups. Despite block randomization, the detemir-only group had proportionally more female participants and fewer male participants than the other two groups (P = 0.007). There was also a modest imbalance in race distribution, with proportionally more black participants in the combination treatment group, although this did not reach significance (P = 0.090). Borderline differences were also seen with respect to glucose and HbA1c (lower in the detemir-only group, P = 0.084 and 0.060, respectively); body fat measurements were available for seven detemir participants (40.6% ± 6.7%), five liraglutide participants (32.6% ± 10.8%), and six combination-treatment participants (41.4% ± 3.8%; P = 0.08 comparing groups). The groups were well balanced with regard to age, weight, BMI, and blood lipid levels (all P > 0.40).
The median metformin dose was 2000 mg/d, with only three participants taking less; all participants were taking at least 1500 mg/d. All participants who were randomly assigned to receive liraglutide and completed the study achieved stable dosing at 1.8 mg/d. The insulin detemir dose was individualized, guided by fasting blood glucose levels as discussed in Methods. The median daily dose was 15 units, with a range from 5 to 68 units per day.
All three treatment groups experienced significant improvements in glycemia over the course of 3 months’ treatment (P = 0.04; Table 2), without a statistical difference between groups (P = 0.12). Weight fell across all three treatment groups (P = 0.007); one individual in the liraglutide group lost 15.7 kg, but otherwise the mean ± SD weight loss was 1.7 ± 2.4 kg and did not differ between treatment groups. Heart rate was unchanged across the course of treatment and not different between groups at the end of treatment (Table 2). Systolic blood pressure fell significantly (P = 0.013); it did not differ between groups (P = 0.28). Diastolic blood pressure also fell significantly (P = 0.006) and this effect differed between groups (P = 0.029), due to larger changes in the detemir-only group.
Table 2.
Treatment Effects
| Variable | Randomization | Month 1 | Month 2 | Study Day | P for Time | P for Treatment × Time |
|---|---|---|---|---|---|---|
| Fasting glucose, mmol/L | 0.04 | 0.12 | ||||
| Detemir | 6.5 ± 2.6 | 7.2 ± 0.9 | 6.9 ± 1.4 | 7.8 ± 2.9 | ||
| Liraglutide | 9.4 ± 3.2 | 6.9 ± 1.1 | 7.2 ± 1.8 | 7.3 ± 2.4 | ||
| Detemir + liraglutide | 9.3 ± 3.1 | 7.2 ± 1.9 | 6.2 ± 0.8 | 6.9 ± 1.4 | ||
| Weight, kg | 0.007 | 0.69 | ||||
| Detemir | 97.4 ± 19.7 | 97.3 ± 20.9 | 96.8 ± 7.0 | 95.7 ± 7.0 | ||
| Liraglutide | 103.1 ± 7.8 | 101.8 ± 7.7 | 100.8 ± 7.5 | 100.1 ± 7.5 | ||
| Detemir + liraglutide | 88.3 ± 8.5 | 86.9 ± 8.3 | 86.9 ± 8.1 | 87.1 ± 8.1 | ||
| Heart rate, bpm | 0.93 | 0.82 | ||||
| Detemir | 69.9 ± 3.3 | 72.7 ± 3.0 | 70.9 ± 3.3 | 69.9 ± 4.0 | ||
| Liraglutide | 72.3 ± 4.4 | 70.3 ± 3.9 | 71.3 ± 4.4 | 74.0 ± 5.3 | ||
| Detemir + liraglutide | 71.3 ± 3.6 | 70.8 ± 3.2 | 75.8 ± 3.6 | 70.8 ± 4.3 | ||
| Systolic BP, mm Hg | 0.013 | 0.28 | ||||
| Detemir | 149.2 ± 6.8 | 129.8 ± 4.4 | 127.8 ± 5.3 | 122.2 ± 7.1 | ||
| Liraglutide | 136.0 ± 8.3 | 135.3 ± 5.3 | 136.0 ± 6.5 | 119.5 ± 8.7 | ||
| Detemir + liraglutide | 131.2 ± 7.4 | 126.8 ± 4.8 | 121.8 ± 5.8 | 126.0 ± 7.8 | ||
| Diastolic BP, mm Hg | 0.006 | 0.029 | ||||
| Detemir | 78.4 ± 2.3 | 71.0 ± 2.0 | 70.9 ± 2.1 | 66.1 ± 2.8 | ||
| Liraglutide | 76.0 ± 2.3 | 70.4 ± 2.0 | 76.1 ± 2.1 | 72.9 ± 5.8 | ||
| Detemir + liraglutide | 73.0 ± 2.5 | 74.0 ± 2.2 | 73.3 ± 2.3 | 72.3 ± 3.0 |
P values are presented for evaluated changes over time for all groups combined (time), and test whether this difference over time differed among treatment groups (treatment × time interaction). BP, blood pressure. Values are mean ± SD.
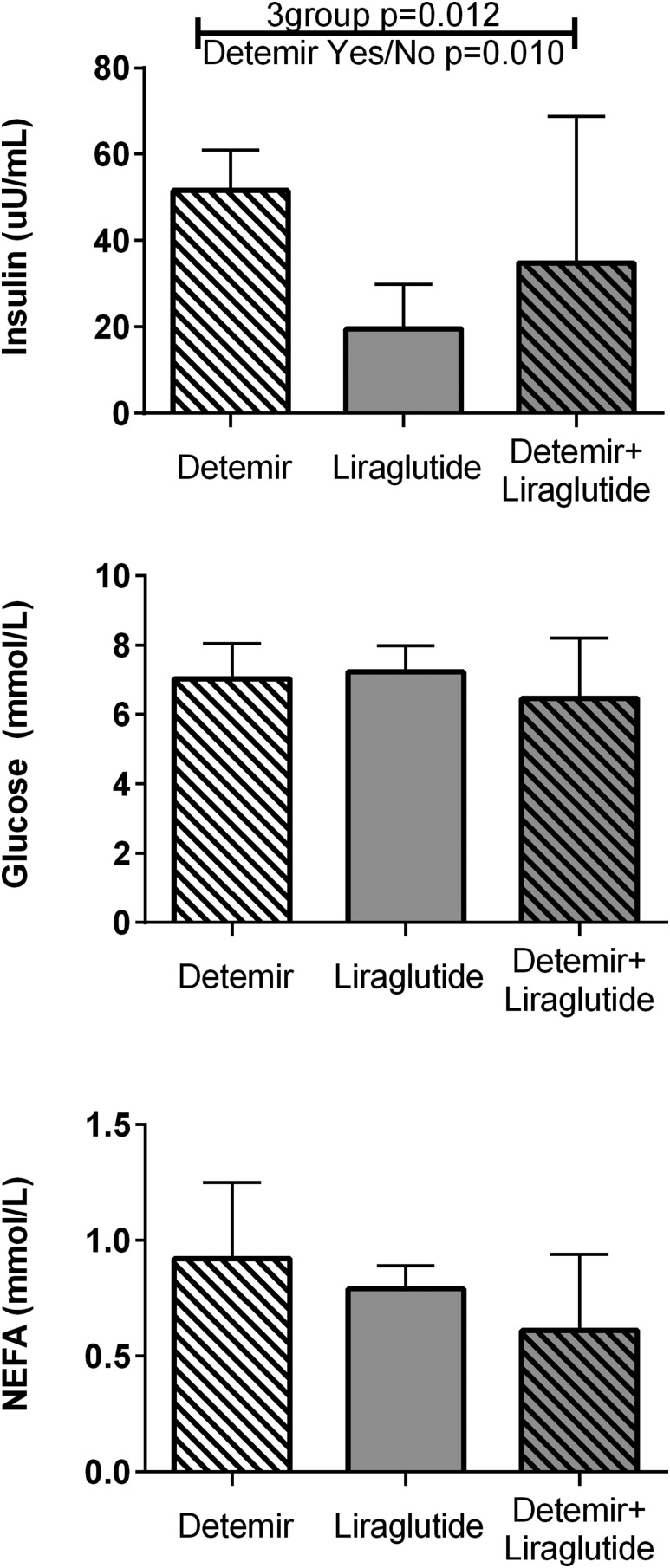
On the day of the PET studies, the three groups did not differ in hemodynamic parameters (Table 2). Importantly, serum glucose values across the interval of PET scanning did not differ between groups (Fig. 2). Nonesterified fatty acid concentrations were numerically but not statistically lower in the detemir plus liraglutide group compared with others (Fig. 2). Insulin concentrations were statistically higher in the participants randomly assigned to detemir alone or to detemir plus liraglutide (Fig. 2), reflecting the steady state from prior treatment plus the 8:00 am dose on the morning of the study.
Figure 2.
Achieved insulin, glucose, and fatty acid concentrations at the time of the PET measurements. Bars represent median values and whiskers represent upper 75th percentile values. Insulin concentrations were significantly different across the three groups, with significance in the pairwise comparison of detemir groups vs. liraglutide alone. The other measures were not significantly different between groups. NEFA, nonesterified fatty acid.
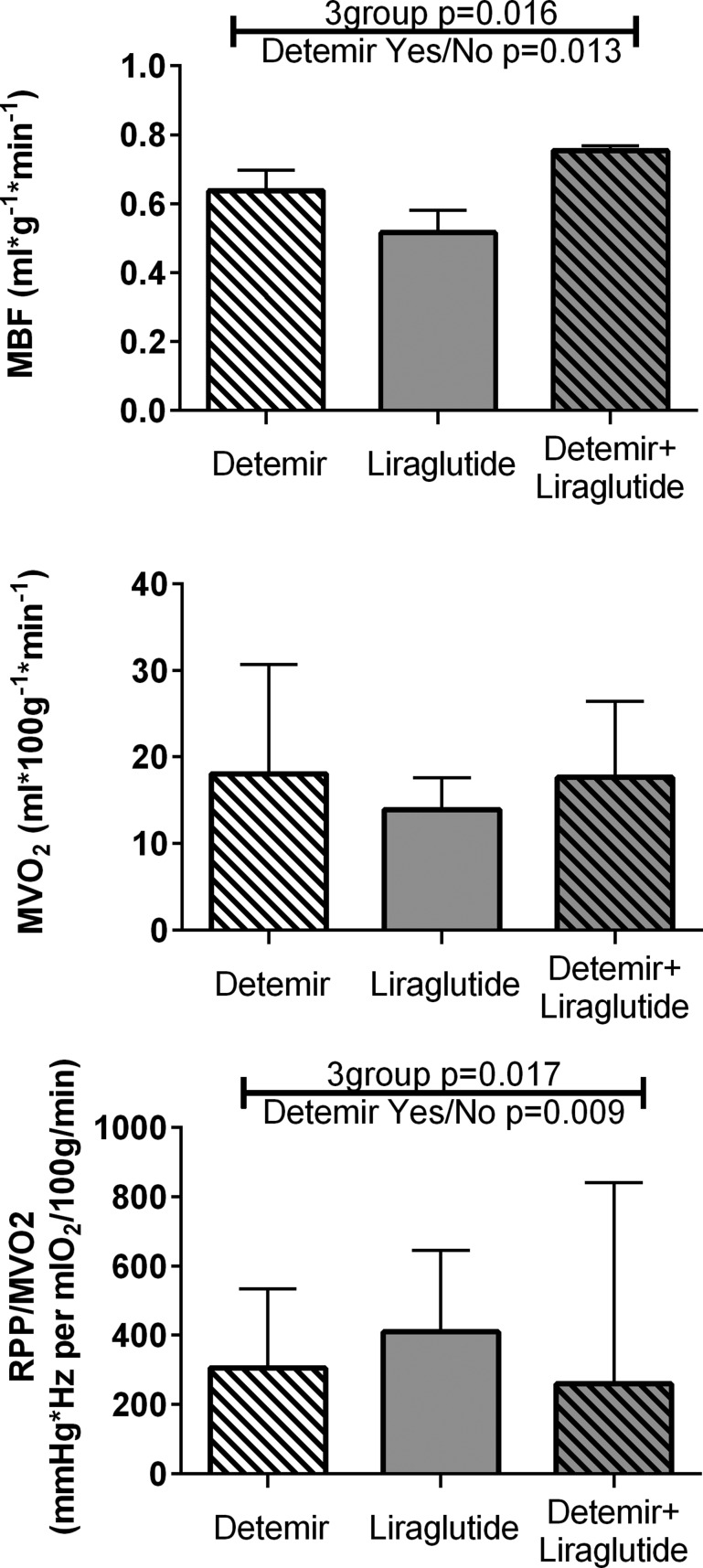
There were no observed differences among groups in the PET-derived measure of MVO2 (Fig. 3). We did observe differences among groups in MBF, with greater rates of blood flow in the two detemir-treated groups compared with the liraglutide-only group. These group differences in blood flow were directionally consistent with the MVO2 group differences, but the correlation between MBF and MVO2 was not statistically significant (r = 0.25, P = 0.22; data not shown). The rate-pressure product (mean arterial pressure multiplied by heart rate) divided by MVO2 was calculated as an index of work efficiency (16, 25) and was statistically greater in the liraglutide-only group compared with the two groups that received insulin (Fig. 3).
Figure 3.
MBF, MVO2, and work efficiency. Bars represent median values and whiskers represent upper 75th percentile values. Only liraglutide alone showed lower blood flow and greater work efficiency than the other two groups. RPP, rate-pressure product.
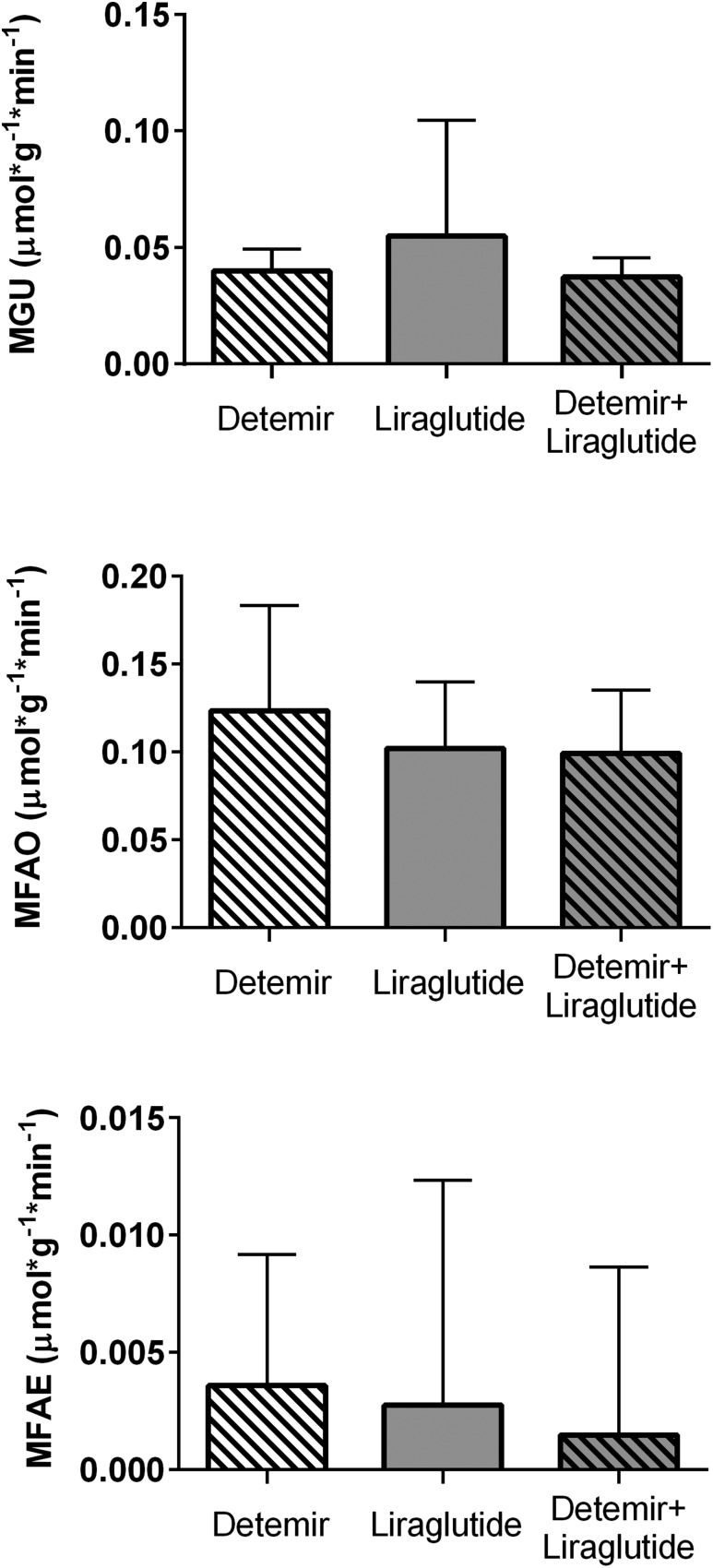
We did not observe any differences between groups in the measures of MGU, fatty acid oxidation, or fatty acid esterification (Fig. 4). The primary hypothesis for this study was that the combination detemir plus liraglutide group would exhibit a higher MGU than detemir alone; this was unequivocally not seen, with these two groups showing essentially identical median measures of MGU (detemir: 0.040 µmol/g/min; detemir plus liraglutide, 0.037 µmol/g/min; P = 0.65; Fig. 4). The liraglutide-only group had a numerically but not statistically higher median MGU (0.055 µmol/g/min; P = 0.57 vs. detemir alone). Similarly, the three treatment groups did not differ in the median values of the palmitate-derived measures of fatty acid use (MFAO median: detemir, 0.123 µmol/g/min; liraglutide: 0.102 µmol/g/min; detemir plus liraglutide, 0.099 µmol/g/min; P = 0.60; Fig. 4). The measured kinetic rates, distinct from substrate availability, were also not different across treatment groups (e.g., glucose kinetic median: detemir, 5.5 × 10−3/min, liraglutide, 8.25 × 10−3/min, combination: 4.65 × 10−3/min, P = 0.69; fatty acid oxidation kinetic: detemir, 165.6 × 10−3/min, liraglutide, 116.4 × 10−3/min, combination, 139.5 × 10−3/min; P = 0.80).
Figure 4.
Myocardial fuel consumption. Bars represent median values and whiskers represent upper 75th percentile values. There were no significant differences across groups in these measures. MFAE, myocardial fatty acid esterification; MFAO, myocardial fatty acid oxidation; MGU, myocardial glucose uptake.
The observed effects on glucose uptake included high outlier values in one detemir-only–treated participant and one liraglutide-only–treated participant. The use of nonparametric statistical testing protects against effects of outliers, but we also performed sensitivity testing excluding these two individuals. Doing so did not alter the outcome (P = 0.84 for MGU comparing three groups). The measures of blood flow, total oxidation, and fatty acid handling from these individuals were not outliers, and parallel sensitivity analyses did not alter any of the comparisons among these other PET-derived parameters.
Sex, race, baseline glucose level, and HbA1c were at least nominally different across treatment groups at baseline (Table 1). However, none of these factors was significantly associated with the PET measures of fuel uptake, with the strongest correlation among these relating HbA1c with myocardial fatty acid esterification (Spearman ρ = −0.35; P = 0.09; most r values <0.10). Sensitivity analyses were performed to further evaluate possible contributions of these imbalances. The results for sex, race, and baseline HbA1c are presented in Supplemental Table 1. Overall, these analyses showed no direct or interaction effect of these variables on the PET-derived outcomes; however, there were two interesting exceptions. First, MGU was significantly different by treatment groups after adjustment for sex: MGU was higher in the liraglutide treatment arm than the other two groups (P < 0.001) and higher in male participants. Second, MFAO was significantly different across treatment groups after adjustment for baseline HbA1c: MFAO was lower in the levemir treatment arm than in the other two groups (P = 0.021), with higher baseline HbA1c relating to higher on-treatment MFAO. This was seen despite equalization of glucose control across the treatment interval before PET measures. These observations do not affect the overall conclusion that combination therapy did not augment fuel use compared with detemir or liraglutide alone.
In summary, treatment with combination detemir plus liraglutide did not produce different rates of fuel use or different patterns of fuel selection compared with insulin alone or liraglutide alone.
Discussion
We measured myocardial fuel use after 12 weeks of randomized therapy with the long-acting insulin detemir, the long-acting GLP-1 mimetic liraglutide, or the combination of these treatments in humans with T2D treated with maximal dose metformin. In vivo measurements of myocardial fuel uptake were made using PET. Study medications were administered the morning of the PET measurements. We observed expected effects of insulin treatment to increase MBF in both groups that received insulin, but otherwise we observed no differences among groups in the rates of fuel metabolism or fuel selection. These data include two distinct observations in T2D without cardiovascular disease: First, liraglutide alone at clinically used doses did not stimulate MGU compared with insulin. Second, liraglutide at clinically used doses did not augment insulin’s effects to drive MGU.
Our primary end point was PET-measured MGU, and our sample size provided a priori power to detect ∼1.2 SD difference in this parameter. We were able to demonstrate expected between-group differences in the effect of insulin on MBF, demonstrating the viability of the overall approach for showing detectable differences. Nevertheless, the results did not suggest any material difference in MGU among groups, with nearly identical group median values and a very high P value comparing the distribution of values. Therefore, although our threshold for demonstrating a change was high, the observed group difference does not suggest that the absence of group differences is a power issue. Similarly, the observed effects on all aspects of fatty acid handling were convincingly equal across groups. These observations do not support the hypothesis that liraglutide alone or in combination with insulin would provide superior stimulation of MGU compared with insulin alone.
Soon after the discovery of GLP-1, this gut peptide was found to exert hemodynamic and metabolic effects in the heart, including protection from the effects of ischemia in mouse models (1, 26–28). Subsequently, a growing body of animal studies shows cardiac benefits of GLP-1 agonists via a variety of mechanisms, including activation of p38α MAPK (11), and improving intracellular calcium homeostasis (29), among others. A major hypothesized mechanism of benefit has been a favorable shift in myocardial metabolite selection induced by GLP-1 agonism (2).
It is interesting to note that the MGU rates were not different across the three treatment groups in our study, despite higher insulin concentrations in the insulin-treated participants compared with those receiving liraglutide alone. This implies that exposure to therapeutic levels of liraglutide provides similar stimulation of MGU, as seen with exposure to therapeutically achieved insulinemia. This could reflect similar direct effects, or similar degrees of resistance to the effects. In this regard we note that the observed levels of MGU were objectively low [consistent with low rates described in prior PET studies in obesity or T2D under fasting conditions (3, 30, 31)], which suggests these observations reflect parallel resistance to the glucose-uptake stimulating effects of insulin and liraglutide in T2D.
In large-scale clinical trials, beneficial cardiovascular effects, including reduced rates of myocardial infarction and death, have been seen with some GLP-1 agonists (e.g., liraglutide, semaglutide) (32, 33) but not others (e.g., lixisenatide, long-acting exenatide) (34, 35). The mechanisms underlying these beneficial effects, or the lack thereof, are unknown. As noted, the antecedent preclinical work suggested that changes in fuel selection might contribute to these effects. However, there are very few studies of effects of GLP-1 agonists on myocardial fuel metabolism in humans. In people with congestive heart failure, liraglutide improved systemic glucose metabolism but failed to improve MGU, MBF, or flow reserve (36). Similarly, albiglutide treatment in people with New York Heart Association class II or III heart failure improved some measures of cardiac function but did not improve MGU or MVO2 (37). Exenatide treatment improved MBF without changing MGU (6), although in that data set a direct relationship was observed between the change in MGU and baseline insulin resistance. The current observations are from a study cohort that was typical of the general population of patients with T2D treated with liraglutide.
We indirectly addressed whether GLP-1 added to insulin could overcome myocardial insulin resistance. There are some data to suggest this is possible. In lean dogs, GLP-1 cotreatment was sufficient to restore insulin-mediated MGU in the setting of chronic heart failure (12). In an observational clinical study, adding liraglutide to ongoing diabetes treatments in T2D for 6 months was associated with improved echocardiographic markers of diastolic function (38). In patients with T2D and known coronary artery disease studied in a prospectively randomized design, echocardiographically measured cardiac function was unaffected by insulin infusion alone but improved with coinfusion of GLP-1 (strain and systolic tissue velocity were significantly improved but ejection fraction was unchanged) (39). One study reported reductions in major atherosclerotic cardiovascular events with adding liraglutide to insulin (40). Collectively, these observations suggest that combination therapy does not detract from hemodynamic or metabolic benefits of individual components, although our current observations suggest that effects on myocardial fuel selection do not explain the observed benefits.
Strengths of this work include the use of typical clinical dosing in the usual target population, randomized assignment to treatment, and the use of high-quality, high-sensitivity measurements of myocardial fuel use with PET. The choice to include metformin as a background treatment condition improves the ability to translate this work to the general circumstance of clinical application of liraglutide but also raises the possibility that our negative results could be due to an unexpected effect of metformin to dominate the apparent treatment response; this seems unlikely given the low absolute rates of MGU overall. Limitations of this work include the relatively low total insulin exposure, which was determined by each individual’s clinical need based on achieved fasting blood glucose levels. We were powered only for demonstrating large between-group differences in our main outcome measure, MGU, but the approximately equal median and distribution across groups argues against a power problem in this finding. It would have been ideal to perform paired measurements of myocardial fuel selection before and after therapy, but this was not possible within the available resources. We performed studies under resting conditions; it is possible that differential responses could be seen under circumstances of physiologic or pathophysiologic demand (i.e., exercise or ischemia). We were not powered a priori for post hoc analyses, so the results of these evaluations need to be considered exploratory. The sex imbalance across treatment groups at baseline might have resulted in imbalanced measures of myocardial fatty acid handling, because women and men differ in this aspect of cardiac fuel selection (41, 42); the sensitivity analysis results suggest the possibility of a difference in response across treatments but do not alter the overall conclusion that combination therapy failed to augment MGU or other measures of fuel selection compared with individual treatments.
In conclusion, the observations from this study suggest liraglutide alone in clinically used dosing in patients with T2D does not induce alterations in resting, fasting myocardial fuel uptake or fuel selection, and that liraglutide was not able to augment the effects of insulin alone on myocardial fuel uptake or selection under these conditions. These observations argue against large effects of GLP-1 agonists on myocardial fuel metabolism as mediators of beneficial treatment effects on myocardial function and ischemia protection.
Supplementary Material
Acknowledgments
Financial Support: This project was supported by an Investigator Initiated Trial grant from Novo Nordisk, and by funding from the National Institutes of Health (Grant HL117620 to J.D.T. and K.J.M.). The Indiana Clinical and Translational Sciences Institute is supported by National Institutes of Health Grants UL1TR001108, TR000006, and RR025671. The sponsors had no access to or influence over the data analyses and did not participate in preparation of the study report.
Clinical Trial Information: ClinicalTrials.gov no. NCT01232946 (registered 2 November 2010).
Author Contributions: K.J.M. and G.D.H conceived the study. K.J.M. drafted the initial manuscript and reviewed and revised the manuscript. C.M., W.T., A.G.G., and J.D.T. contributed to the data analysis. R.V.C., L.H., N.A.P., M.A.G., and G.D.H. collected data, contributed to the discussion, and reviewed the manuscript. All authors approved the final manuscript as submitted. K.J.M. is the guarantor of this work.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- GLP-1
glucagon-like peptide-1
- MBF
myocardial blood flow
- MFAO
myocardial fatty acid oxidation
- MGU
myocardial glucose uptake
- MVO2
myocardial oxygen consumption
- PET
positron emission tomography
- T2D
type 2 diabetes mellitus
References
- 1. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–870. [DOI] [PubMed] [Google Scholar]
- 2. Aravindhan K, Bao W, Harpel MR, Willette RN, Lepore JJ, Jucker BM. Cardioprotection resulting from glucagon-like peptide-1 administration involves shifting metabolic substrate utilization to increase energy efficiency in the rat heart. PLoS One. 2015;10(6):e0130894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moberly SP, Mather KJ, Berwick ZC, Owen MK, Goodwill AG, Casalini ED, Hutchins GD, Green MA, Ng Y, Considine RV, Perry KM, Chisholm RL, Tune JD. Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic Res Cardiol. 2013;108(4):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sassoon DJ, Tune JD, Mather KJ, Noblet JN, Eagleson MA, Conteh AM, Sturek JT, Goodwill AG. Glucagon-like peptide 1 receptor activation augments cardiac output and improves cardiac efficiency in obese swine after myocardial infarction. Diabetes. 2017;66(8):2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gejl M, Lerche S, Mengel A, Møller N, Bibby BM, Smidt K, Brock B, Søndergaard H, Bøtker HE, Gjedde A, Holst JJ, Hansen SB, Rungby J. Influence of GLP-1 on myocardial glucose metabolism in healthy men during normo- or hypoglycemia. PLoS One. 2014;9(1):e83758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gejl M, Søndergaard HM, Stecher C, Bibby BM, Møller N, Bøtker HE, Hansen SB, Gjedde A, Rungby J, Brock B. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(7):E1165–E1169. [DOI] [PubMed] [Google Scholar]
- 7. Moberly SP, Berwick ZC, Kohr M, Svendsen M, Mather KJ, Tune JD. Intracoronary glucagon-like peptide 1 preferentially augments glucose uptake in ischemic myocardium independent of changes in coronary flow. Exp Biol Med (Maywood). 2012;237(3):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51(10):3020–3024. [DOI] [PubMed] [Google Scholar]
- 9. Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovasc Res. 2008;79(2):238–248. [DOI] [PubMed] [Google Scholar]
- 10. Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53(9):2366–2374. [DOI] [PubMed] [Google Scholar]
- 11. Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3(4):512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen M, Angeli FS, Shen YT, Shannon RP. GLP-1 (7-36) amide restores myocardial insulin sensitivity and prevents the progression of heart failure in senescent beagles. Cardiovasc Diabetol. 2014;13(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buck A, Wolpers HG, Hutchins GD, Savas V, Mangner TJ, Nguyen N, Schwaiger M. Effect of carbon-11-acetate recirculation on estimates of myocardial oxygen consumption by PET. J Nucl Med. 1991;32(10):1950–1957. [PubMed] [Google Scholar]
- 14. Ng Y, Moberly SP, Mather KJ, Brown-Proctor C, Hutchins GD, Green MA. Equivalence of arterial and venous blood for [11C]CO2-metabolite analysis following intravenous administration of 1-[11C]acetate and 1-[11C]palmitate. Nucl Med Biol. 2013;40(3):361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hutchins GD, Chen T, Carlson KA, Fain RL, Winkle W, Vavrek T, Mock BH, Zipes DP. PET imaging of oxidative metabolism abnormalities in sympathetically denervated canine myocardium. J Nucl Med. 1999;40(5):846–853. [PubMed] [Google Scholar]
- 16. Mather KJ, Hutchins GD, Perry K, Territo W, Chisholm R, Acton A, Glick-Wilson B, Considine RV, Moberly S, DeGrado TR. Assessment of myocardial metabolic flexibility and work efficiency in human type 2 diabetes using 16-[18F]fluoro-4-thiapalmitate, a novel PET fatty acid tracer. Am J Physiol Endocrinol Metab. 2016;310(6):E452–E460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang SC, Kofoed KF, Weismueller S, Czernin J, Phelps ME, Schelbert HR. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11]acetate. J Nucl Med. 1998;39(2):272–280. [PubMed] [Google Scholar]
- 18. de Jong HW, Rijzewijk LJ, Lubberink M, van der Meer RW, Lamb HJ, Smit JW, Diamant M, Lammertsma AA. Kinetic models for analysing myocardial [(11)C]palmitate data. Eur J Nucl Med Mol Imaging. 2009;36(6):966–978. [DOI] [PubMed] [Google Scholar]
- 19. Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37(10):1723–1730. [PubMed] [Google Scholar]
- 20. Morita K, Katoh C, Yoshinaga K, Noriyasu K, Mabuchi M, Tsukamoto T, Kageyama H, Shiga T, Kuge Y, Tamaki N. Quantitative analysis of myocardial glucose utilization in patients with left ventricular dysfunction by means of 18F-FDG dynamic positron tomography and three-compartment analysis. Eur J Nucl Med Mol Imaging. 2005;32(7):806–812. [DOI] [PubMed] [Google Scholar]
- 21. Bøtker HE, Böttcher M, Schmitz O, Gee A, Hansen SB, Cold GE, Nielsen TT, Gjedde A. Glucose uptake and lumped constant variability in normal human hearts determined with [18F]fluorodeoxyglucose. J Nucl Cardiol. 1997;4(2 Pt 1):125–132. [DOI] [PubMed] [Google Scholar]
- 22. Bøtker HE, Goodwin GW, Holden JE, Doenst T, Gjedde A, Taegtmeyer H. Myocardial glucose uptake measured with fluorodeoxyglucose: a proposed method to account for variable lumped constants. J Nucl Med. 1999;40(7):1186–1196. [PubMed] [Google Scholar]
- 23. van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119(15):2069–2077. [DOI] [PubMed] [Google Scholar]
- 24. Hällsten K, Virtanen KA, Lönnqvist F, Janatuinen T, Turiceanu M, Rönnemaa T, Viikari J, Lehtimäki T, Knuuti J, Nuutila P. Enhancement of insulin-stimulated myocardial glucose uptake in patients with type 2 diabetes treated with rosiglitazone. Diabet Med. 2004;21(12):1280–1287. [DOI] [PubMed] [Google Scholar]
- 25. Hattori N, Tamaki N, Kudoh T, Masuda I, Magata Y, Kitano H, Inubushi M, Tadamura E, Nakao K, Konishi J. Abnormality of myocardial oxidative metabolism in noninsulin-dependent diabetes mellitus. J Nucl Med. 1998;39(11):1835–1840. [PubMed] [Google Scholar]
- 26. Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317(3):1106–1113. [DOI] [PubMed] [Google Scholar]
- 27. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54(1):146–151. [DOI] [PubMed] [Google Scholar]
- 28. Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu SY, Zhang Y, Zhu PJ, Zhou H, Chen YD. Liraglutide directly protects cardiomyocytes against reperfusion injury possibly via modulation of intracellular calcium homeostasis. J Geriatr Cardiol. 2017;14(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Botker HE, Moller N, Schmitz O, Bagger JP, Nielsen TT. Myocardial insulin resistance in patients with syndrome X. J Clin Invest. 1997;100(8):1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, Sasaki Y. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36(3):456–463. [PubMed] [Google Scholar]
- 32. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; for the LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 34. Derosa G, Maffioli P. Lixisenatide in type 2 diabetes and acute coronary syndrome. N Engl J Med. 2016;374(11):1095. [DOI] [PubMed] [Google Scholar]
- 35. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nielsen R, Jorsal A, Iversen P, Tolbod LP, Bouchelouche K, Sørensen J, Harms HJ, Flyvbjerg A, Tarnow L, Kistorp C, Gustafsson I, Bøtker HE, Wiggers H. Effect of liraglutide on myocardial glucose uptake and blood flow in stable chronic heart failure patients: a double-blind, randomized, placebo-controlled LIVE sub-study [published online ahead of print 2 August 2017] J Nucl Cardiol. doi: 10.1007/s12350-017-1000-2. [DOI] [PubMed]
- 37. Lepore JJ, Olson E, Demopoulos L, Haws T, Fang Z, Barbour AM, Fossler M, Davila-Roman VG, Russell SD, Gropler RJ. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail. 2016;4(7)559–556 [DOI] [PubMed] [Google Scholar]
- 38. Saponaro F, Sonaglioni A, Rossi A, Montefusco L, Lombardo M, Adda G, Arosio M. Improved diastolic function in type 2 diabetes after a six month liraglutide treatment. Diabetes Res Clin Pract. 2016;118:21–28. [DOI] [PubMed] [Google Scholar]
- 39. McCormick LM, Heck PM, Ring LS, Kydd AC, Clarke SJ, Hoole SP, Dutka DP. Glucagon-like peptide-1 protects against ischemic left ventricular dysfunction during hyperglycemia in patients with coronary artery disease and type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anyanwagu U, Mamza J, Donnelly R, Idris I. Effect of adding GLP-1RA on mortality, cardiovascular events, and metabolic outcomes among insulin-treated patients with type 2 diabetes: a large retrospective UK cohort study. Am Heart J. 2018;196:18–27. [DOI] [PubMed] [Google Scholar]
- 41. Kadkhodayan A, Lin CH, Coggan AR, Kisrieva-Ware Z, Schechtman KB, Novak E, Joseph SM, Dávila-Román VG, Gropler RJ, Dence C, Peterson LR. Sex affects myocardial blood flow and fatty acid substrate metabolism in humans with nonischemic heart failure. J Nucl Cardiol. 2017;24(4):1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging. 2008;1(4):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.