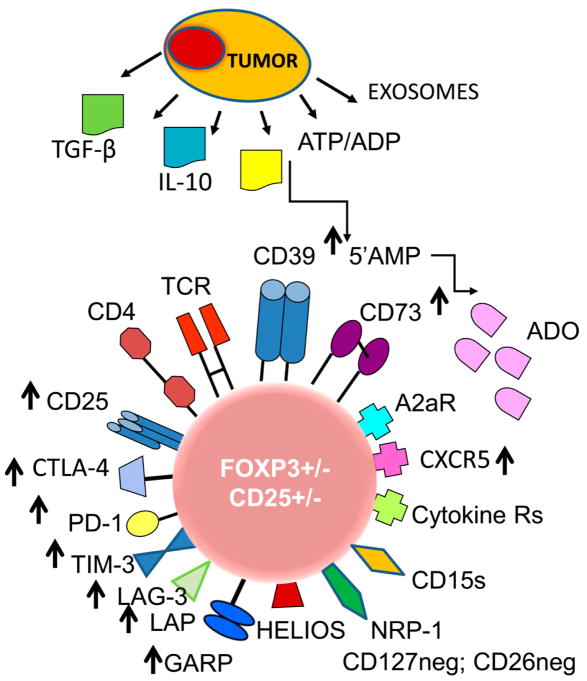
Figure 1.
The phenotypic profile of an iTreg in the tumor microenvironment (TME). The tumor produces cytokines, soluble factors or exosomes, all of which re-program functions of Treg in the TME. Highly activated iTreg up-regulate expression levels of checkpoint inhibitory receptors and other proteins involved in immunosuppressive activity of iTreg, such as TGF-β-associated LAP and GARP or CD39 and CD73 ectoenzymes (see arrows). iTreg in the TME may or may not be FOXP3+ and CD25+; they up-regulate expression levels of NRP1, which maintains contact with DCs via the semaphorin-4a and may play a role in Treg stability. Abbreviations: Treg, regulatory T cells, ATP, adenosine triphosphate, ADP, adenosine diphosphate; 5’AMP, adenosine-5’-monophosphate; ADO, adenosine; TCR, T-cell receptor; CTLA-4, cytotoxic lymphocyte antigen-4; PD-1, programmed death-1; TIM-3, T-cell immunoglobulin mucin-3; LAG-3, lymphocyte activation gene-3; TGF-β, transforming growth factor- beta; LAP, latency-associated protein; GARP, glycoprotein A repetition predominant; NRP-1, neuoropilin-1; CD15s, sialyl Lewis X.