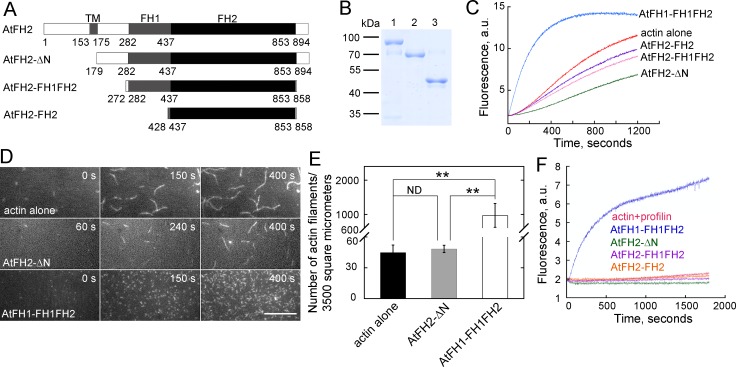
Figure 5. AtFH2 fails to nucleate actin assembly from actin or actin bound to profilin, and slightly inhibits spontaneous actin polymerization in vitro.
(A) Schematic representation of the domain organization of AtFH2 and the fragments used for the generation of recombinant truncated AtFH2 proteins. (B) Purified recombinant truncated AtFH2 proteins. The recombinant AtFH2 proteins were resolved on 10% SDS-PAGE and stained with Coomassie blue. Lane 1, AtFH2-ΔN; lane 2, AtFH2-FH1FH2; lane 3, AtFH2-FH2. (C) The effect of AtFH2 on spontaneous actin assembly from G-actin alone. Actin (10% pyrene-labeled, 3 μM) was incubated with recombinant formin proteins (1 μM) for 5 min at room temperature before the addition of 10 × KMEI to initiate actin polymerization, and actin polymerization was traced by monitoring the changes in pyrene fluorescence. (D) Time-lapse images of actin filaments in the absence or presence of formin proteins. [Actin], 1.5 μM (33.3% Oregon Green-labeled); [AtFH2-ΔN], 400 nM; [AtFH1-FH1FH2], 100 nM. Bar = 10 μm. (E) Quantification of the number of actin filaments per microscope field. Values represent mean ± SE, n = 3. **p<0.01 by Student’s t-test. (F) AtFH2 fails to utilize profilin-actin complexes. Actin (10% pyrene-labeled, 3 μM) plus human profilin I (3 μM) were incubated with recombinant formin proteins (1 μM) for 5 min at room temperature before the addition of 10 × KMEI to initiate actin polymerization, and actin polymerization was traced by monitoring the changes in pyrene fluorescence. AtFH1-FH1FH2 (Michelot et al., 2005) was used as the control in (C) and (F).