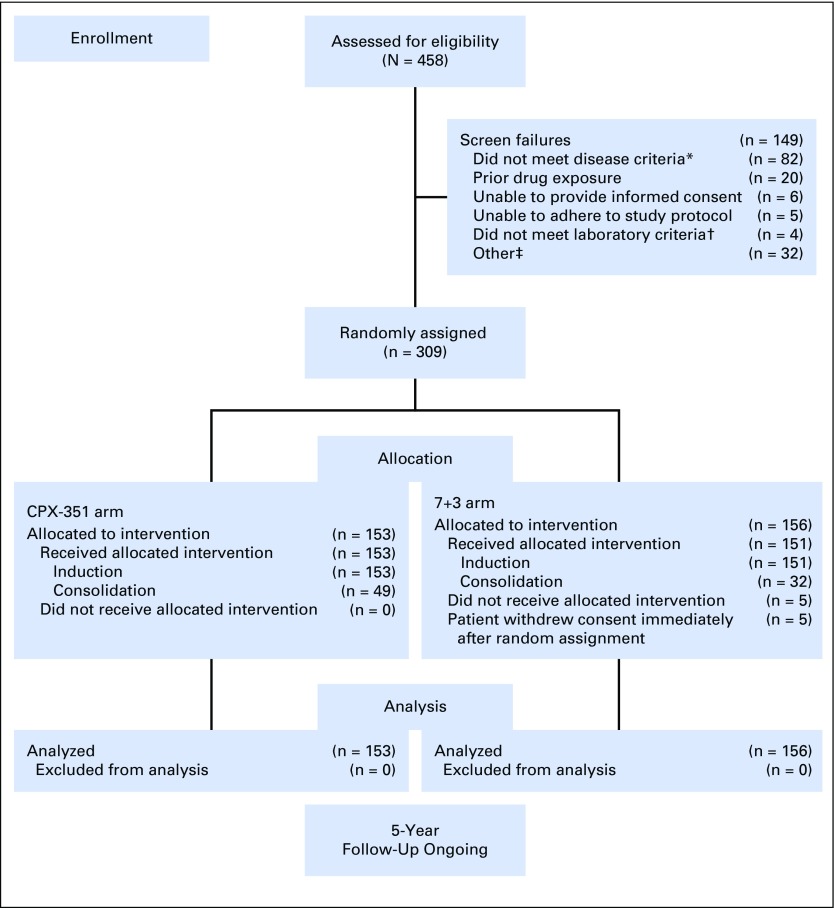
Fig 1.
CONSORT diagram. (*) Patients without confirmation of therapy-related acute myeloid leukemia (AML), antecedent myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML), or de novo AML with MDS-related cytogenetic abnormalities (n = 43; many of these screen failures were in patients with de novo AML without MDS-related cytogenetic abnormalities because results of the required bone marrow biopsy specimens were not available at the patients’ initial diagnosis); without pathologic diagnosis of AML according to WHO criteria with ≥ 20% blasts in the peripheral blood or bone marrow (n = 30); with a history of myeloproliferative neoplasms, except CMML (n = 6); or with acute promyelocytic leukemia (t[15;17]) or favorable cytogenetics (t[8;21] or inv16 if known at the time of random assignment; n = 3). (†) Patients without serum creatinine < 2.0 mg/dL, serum total bilirubin < 2.0 mg/dL, and serum ALT or AST less than three times the upper limit of normal. (‡) Other includes patients with myocardial impairment of any cause that resulted in heart failure by New York Heart Association criteria (n = 3); active (uncontrolled, metastatic) second malignancies (n = 1); active fungal infection, hepatitis B or C, or HIV (n = 1); cardiac ejection fraction < 50% (n = 1); incorrect age (n = 1); secondary malignancy in remission (n = 1); and unspecified (n = 24). 7+3, standard-of-care cytarabine plus daunorubicin chemotherapy; CPX-351, dual-drug liposomal encapsulation of cytarabine and daunorubicin.