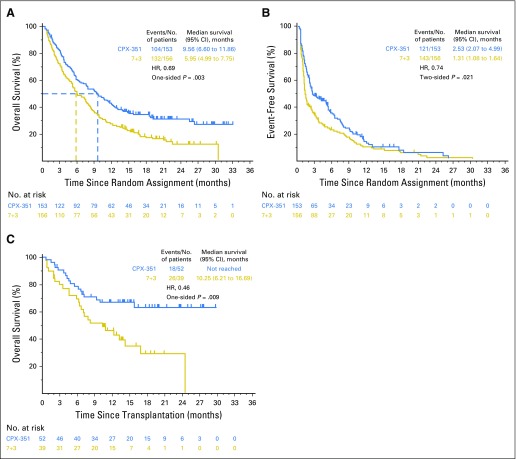
Fig 2.
Median overall survival (OS) and event-free survival (EFS). Kaplan-Meier estimates of (A) OS and (B) EFS are shown for the overall intention-to-treat population, and Kaplan-Meier estimates of (C) OS landmarked from the date of transplantation are shown for patients in the intention-to-treat population who received a hematopoietic cell transplant. EFS was defined as the time from random assignment to the date of induction treatment failure, relapse from complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi), or death as a result of any cause, whichever came first. Patients alive and not known to have any of these events were censored on the latter of their last dates of disease assessment or hematology assessment. For patients who achieved CR or CRi, duration of remission was measured from the date of remission (CR or CRi) until the date of relapse or death as a result of any cause. Patients not known to have relapsed or died at the last follow-up were censored in a similar fashion as described for EFS. 7+3, standard-of-care cytarabine plus daunorubicin chemotherapy; CPX-351, dual-drug liposomal encapsulation of cytarabine and daunorubicin; HR, hazard ratio.