Abstract
Two well-preserved specimens of a new eosauropterygian from the Upper Triassic of Central Spain are attributed to a new taxon, Paludidraco multidentatus gen. et sp. nov. It is a member of Simosauridae that presents several exclusive characters suggesting a highly specialized trophic adaptation. This discovery increases the already high ecological disparity of the Triassic marine reptiles.
Keywords: Sauropterygia, Simosauridae, Keuper, Spain
1. Introduction
Sauropterygia is a group of successful aquatic reptiles that appeared in the Early Triassic and expanded in range throughout the Mesozoic, and demonstrated considerable diversity in form and size during the Triassic [1]. Some became enormous predators [2], whereas others developed alternative lifestyles, such as in the case of the durophagous or herbivorous placodonts [3].
Nothosauria is a well-represented and diverse Eurasian Triassic sauropterygian clade [2]. Its sister group, Simosauridae, is poorly known, only being represented by the Central European Middle Triassic Simosaurus gaillardoti [4]. A relatively complete skeleton and a second skull of a new simosaurid from the Upper Triassic of Central Spain, Paludidraco multidentatus gen. et. sp. nov., are presented here.
Simosaurus gaillardoti was as an active predator with large and blunt conical teeth, ideal for capturing and feeding on relatively hard prey [4]. The axial skeleton of the new simosaurid is much more pachyostotic than that of any known middle-to-large Triassic sauropterygian. Indeed, it comprises highly specialized gracile skull and jaws, with numerous small teeth. These reflect a pleurodont attachment that can be contrasted with the thecodont condition of the other unambiguous sauropterygians. Thus, P. multidentatus played an ecological role as yet unknown in the disparity of the successful clade Sauropterygia.
1.1. Institutional abbreviations
MUPA-ATZ, El Atance collection, Museo de Paleontología de Castilla-La Mancha (Cuenca, Spain). MNHN, Muséum national d'Histoire naturelle (Paris, France). SMNS, Staatliches Museum für Naturkunde Stuttgart (Stuttgart, Germany).
1.2. Anatomical abbreviations
a, angular; adr, anterior dorsal rib; ar, articular; bo, basioccipital; c, coronoid; cl, clavicle; co, coracoid; cv, caudal vertebra; d, dentary; dr, dorsal rib; dv, dorsal vertebrae; ec, ectopterygoid; f, frontal; fe, femur; i, ilion; j, jaw; ju, jugal; ldv, last dorsal vertebra; lr, lumbar rib; m, maxilla; n, nasal; p, parietal; pl, palatine; pm, premaxilla; po, postorbital; pof, postfrontal; prf, prefrontal; pt, pterigoyd; pu, pubis; q, quadrate; qj, quadratojugal; s, splenial; sa, surangular; sc, scapula; sk, skull; so, supraoccipital; sq, squamosal; sr, sacral rib; sv, sacral vertebra; v, vomer.
2. Methodology
The comparison between P. multidentatus and S. gaillardoti is based in [4] and in the personal observation of the Simosaurus material kept in MNHN and SMNS. Paludidraco multidentatus was coded in the modified version of the matrix of Neenan et al. [5] proposed by Cheng et al. [6], with several modifications (see the electronic supplementary material).
The data matrix is composed of 50 taxa and 144 characters. It was analysed using TNT 1.0 [7]. A traditional search was used, with 1000 replications of Wagner trees, followed by tree bisection recognition as a swapping algorithm, saving 100 trees per replication.
3. Systematic palaeontology
Sauropterygia Owen, 1860
Eosauropterygia Rieppel, 1994
Nothosauroidea Baur, 1889
Simosauridae Huene, 1948
Type species. Simosaurus gaillardoti Meyer, 1842
Included species. Simosaurus gaillardoti, Paludidraco multidentatus gen. et. sp. nov.
Emended diagnosis. Nothosauroidea with the following synapomorphies according to the phylogenetic results obtained here: mandibular articulations displaced to a level distinctly behind occipital condyle; vertebral centra distinctly constricted in ventral view; distinct expansion of distal head of sacral ribs; infraprezygapophyses and infrapostzygapophyses at least in the dorsal, sacral and most anterior caudal vertebrae and anterolateral process of the clavicle. In addition, it differs from Nothosauria by: relatively short and blunt snout, without rostral constriction; unreduced prefrontals; jugal extending anteriorly along the lateral margin of the orbit; undepressed temporal region; absence of premaxillary and dentary fangs and absence of enlarged teeth on the maxilla.
Distribution. Middle to Upper Triassic (Anisian to Norian) of Europe and Middle East [4,8,9].
Paludidraco multidentatus gen. et sp. nov.
Figure 1.
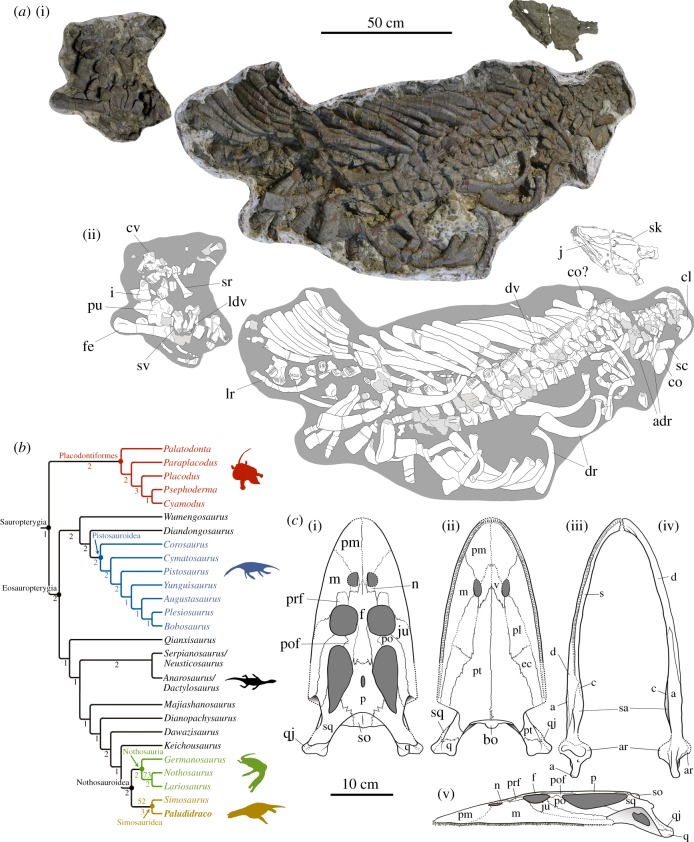
(a) MUPA-ATZ0101, holotype of the new eosauropterygian Paludidraco multidentatus. The relative position of the two blocks and the skull corresponds to their original position in the field. (b) Phylogenetic position. Bootstrap frequencies exceeding 50 per cent (top) and Bremer values (bottom) are indicated. (c) Reconstruction of the skull (c(i), c(ii) and c(v)) and jaws (c(iii) and c(iv)) of P. multidentatus in dorsal (c(i) and c(iii)), ventral (c(ii) and c(iv)) and lateral (c(v)) views.
Figure 2.
Cranial remains of the holotype (a–e; MUPA-ATZ010) and paratype (f–i; MUPA-ATZ0102) of the new eosauropterygian Paludidraco multidentatus. (a,b) Skull in dorsal (a) and ventral (b) views. (c,d) Posterior region of the left mandibular rami in lateral (c) and lingual (d) views. (e) Detail of a tooth. (f,g) Skull and left mandibular rami in dorsal (f) and ventral (g) views. (h) Detail of the mandibular symphysis in dorsal view. (i) Detail of the teeth attachment area in the premaxilla and maxilla, in dorsal view.
Type material. Holotype, MUPA-ATZ0101, a relatively complete and partially articulated skeleton, including the skull, both incomplete mandibular rami, most of the dorsal vertebrae and ribs, some sacral and caudal vertebrae, and elements of the girdles and the appendicular regions (figures 1a and 2a–e). Paratype, MUPA-ATZ0102, a skull and most of the left jaw (figure 2f–i; electronic supplementary material).
Type locality and type horizon. El Atance site (Sigüenza, Guadalajara, Central Spain). Keuper Facies. Carnian-Norian (Upper Triassic) [10].
Etymology. The generic name is composed of the Latin words paludos, meaning marshy (in reference to the El Atance reservoir) and draco, from the Latin word for dragon. The specific name refers to the Latin words multi, meaning numerous, and dentatus, for toothed.
Diagnosis. Simosauridae with the following autapomorphies according to the phylogenetic results obtained here: teeth inserted in pleurodont implantation and more than 15 premaxillary teeth. In addition, it differs from Simosaurus gaillardoti by: relatively elongated snout; retracted external nares; narrow upper temporal fenestra; elongated pineal foramen, close to the middle area of the skull table; horizontal exposition of the supraoccipital at the posterior end of the parietal table; supraoccipital horizontally sutured with the parietal; very slender and fragile jaw; splenial located dorsally to the dentary; teeth situated laterally in the jaw; numerous, small, recurved and mesiodistally compressed teeth; amphicoelous centra; pachyostotic zygapophyses; pachyostotic ribs and strong distal expansion of the dorsal ribs.
4. Discussion
The phylogenetic analyses resulted in four most parsimonious trees, with a length of 634 steps (CI = 0.311; RI = 0.664). The strict consensus tree replicates the topology obtained by Cheng et al. [6]. Paludidraco multidentatus is grouped with S. gaillardoti, comprising Simosauridae (i.e. the sister clade of Nothosauria) (figure 1b and electronic supplementary material).
The teeth and jaw of P. multidentatus show a configuration never before seen in Sauropterygia. The jaw is very slender and weak, with bowed mandibular rami and lacking a reinforced symphysis. In addition, the premaxillae, the maxillae and the dentary carry numerous small and curved teeth, mesiodistally compressed and inserted in a pleurodont implantation, which contrasts with the thecodont disposition in other eosauropterygians [11]. The Chinese Middle Triassic Atopodentatus unicus, interpreted as being related to Sauropterygia or as a basal sauropterygian, also shows pleurodonty [12]. Paludidraco multidentatus is not interpreted as an active predator: its gracile cranial structure would have precluded the capture of prey that could offer resistance, and its robust and pachyostotic post-cranial skeleton would have restricted its maneuverability.
The slender and bowed mandibles and the numerous small teeth form a comb-like structure, which suggests filter-feeding. This was also proposed for the Late Cretaceous plesiosaur Morturneria seymourensis [13], based on its dense battery of small teeth, bowed mandibles that lack a reinforced symphysis, quadrates lying far behind the occipital condyle and deeply arched palate forming a big oral cavity. Both taxa share most of these characters, but P. multidentatus lacks the deeply arched palate, and its mandible is even more slender and gracile than that of M. seymourensis. According to the studies of Collin & Janis [14] and Motani et al. [15], P. multidentatus lacks some features necessary to perform successful suspension filter-feeding. Suspension feeder marine tetrapods (i.e. whales and the proposed filter feeder Hupehsuchus) have slender and bowed jaws, and the mandibular rami are not fused, facilitating their rotation and the expansion of the oral cavity when huge amounts of water are swallowed [15]. In addition, whales exhibit other characters that have been proposed as unique to filter feeders, including small eyes, very elongated rostra and large skulls (from a quarter to a third of the total length of the body) [14]. All of these conditions are absent in P. multidentatus and thus counter its interpretation as a suspension feeder. Their shared absence in the putative filter feeder M. seymourensis suggests the evolution of other modalities of feeding by filtration in Sauropterygia. Although neither the possible sauropterygian A. unicus nor the placodont Henodus chelyops fit into the category of suspension feeders, it has been suggested that they acquired their food by filter-feeding [12]. Henodus chelyops is interpreted as a non-marine placodont that cut or scraped plant material using small denticles of the premaxillae edge and swallowed it via an extensible throat [3,16]. The filtration would have been performed through hypothetical non-ossified baleen-like maxillary and dentary structures [16]. Atopodentatus unicus has been suggested as the earliest herbivore marine reptile, with the premaxillae, dentaries and maxillae arranged in a T-shape and the row of premaxillary pleurodont teeth scraping the plants off the substrate [12]. This vegetal material would have been gulped into the oral cavity via a rapid opening of the mouth. Water would have been forced out by the tongue after the mouth was closed, and the dense palisade of needle-like teeth would have filtered the food [12]. Therefore, both H. chelyops and A. unicus have been interpreted as capable of performing filtration, in spite of not being suspension feeders.
The slender jaws and curved and flat teeth of P. multidentatus appear to have been inadequate for cutting or scraping submarine plants with a resistant vegetative apparatus, in contrast with H. chelyops and A. unicus. However, it could also have fed on plants, browsing soft vegetal material from the bottom. It is also plausible that P. multidentatus captured small animals from the substrate. In both circumstances, it would swallow the food and the water from the bottom, before filtering it with the comb-like dental structure.
In addition to its peculiar cranial structure, P. multidentatus is characterized by its extremely pachyostotic axial skeleton, unregistered in any other Triassic sauropterygian of middle or large size [17,18]. The proposed filter feeders Morturneria, Atopodentatus and Henodus are not pachyostotic.
Pachyostotic aquatic amniotes are recognized as inhabitants of shallow marine environments [17,18]. Although pachyostosis implies limited acceleration and maneuverability, it enables the attainment of neutral buoyancy at shallow depths, floating without expending energy or walking and searching for food on the bottom, akin to the sirenians [17,18]. Given the extremely pachyostotic post-cranial skeleton of P. multidentatus, we propose a lifestyle similar to that of these mammals, swimming slowly near the marine bottom in shallow waters and feeding on soft plant material or small and soft animals.
5. Conclusion
Remains belonging to two individuals of the new eosauropterygian Paludidraco multidentatus gen. et sp. nov. have been recovered from the Upper Triassic El Atance site (Central Spain). Paludidraco multidentatus is primarily characterized by a very slender mandible and numerous small and sickle-like teeth with pleurodont implantation, as well as an extremely pachyostotic axial skeleton. An ecological role analogous to that of extant manatees is interpreted for this bizarre sauropterygian. Therefore, this discovery increases the already high disparity of the sauropterygians from the Triassic.
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank P. Castrillo, M. Onrubia, A. Santiandreu, N. Torres and specially F. Marcos for preparation; P. Mocho, A. Páramo and D. Vidal for photographs; J.M. Quesada for his inestimable collaboration; the Confederación Hidrográfica del Tajo (especially O. Cabrera) for the support; N.E. Jalil and R. Schoch for access to comparative collections and the editor S. Johar, the reviewer P.M. Sander and an anonymous reviewer for their kind comments, which have helped to improve this paper.
Ethics
All specimens are appropriately deposited in accordance with the ICZN, and all permissions to carry out this research were obtained.
Data accessibility
Figures, data matrix modifications, the data matrix file and information on Simosauridae are available as the electronic supplementary material.
Authors' contributions
All authors contributed in an identical way in the elaboration of this manuscript. All authors gave final approval for publication and agree to be accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
This research was funded by a FPI UNED Grant (0271864713 Y0SC001170), two SYNTHESYS projects (FR-TAF-5710 and DE-TAF-5955) and the Consejería de Educación, Cultura y Deportes of Castilla-La Mancha (SBPLY/15/180601/000044).
References
- 1.Stubbs TL, Benton MJ. 2016. Ecomorphological diversifications of Mesozoic marine reptiles: the roles of ecological opportunity and extinction. Paleobiology 42, 547–573. ( 10.1017/pab.2016.15) [DOI] [Google Scholar]
- 2.Liu J, et al. 2014. A gigantic nothosaur (Reptilia: Sauropterygia) from the Middle Triassic of SW China and its implication for the Triassic biotic recovery. Sci. Rep. 4, 1–9. ( 10.1038/srep07142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieppel O. 2002. Feeding mechanics in Triassic stem-group sauropterygians: the anatomy of a successful invasion of Mesozoic seas. Zool. J. Linn. Soc. 135, 33–63. ( 10.1046/j.1096-3642.2002.00019.x) [DOI] [Google Scholar]
- 4.Rieppel O. 1994. Osteology of Simosaurus gaillardoti and the relationships of stem-group Sauropterygia. Fieldiana Geol. 28, 85. [Google Scholar]
- 5.Neenan JM, Klein N, Scheyer TM. 2013. European origin of placodont marine reptiles and the evolution of crushing dentition in Placodontia. Nat. Commun. 4, 1621 ( 10.1038/ncomms2633) [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y, Wu X, Sato T, Shan H. 2016. Dawazisaurus brevis, a new eosauropterygian from the Middle Triassic of Yunnan, China. Acta Geol. Sin. Engl. 90, 401–424. ( 10.1111/1755-6724.12680) [DOI] [Google Scholar]
- 7.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. ( 10.1111/j.1096-0031.2008.00217.x) [DOI] [Google Scholar]
- 8.Vecchia FM D. 2008. First record of Simosaurus (Sauropterygia. Nothosauroidea) from the Carnian (Late Triassic) of Italy. Riv. Ital. Paleontol. Stratigrafia 114, 273–285. [Google Scholar]
- 9.de Miguel Chaves C, Pérez-García A, Cobos A, Royo-Torres R, Ortega F, Alcalá L. 2015. A diverse Late Triassic tetrapod fauna from Manzanera (Teruel, Spain). Geobios 48, 479–490. ( 10.1016/j.geobios.2015.09.002) [DOI] [Google Scholar]
- 10.Adell Argiles F, Bascones Alvira L, Martínez Álvarez N, Tena-Dávila Ruiz M, González Lodeiro F, La Moneda González E, Rodríguez González A. 1981. Hoja geológica num. 461 (Sigüenza). Mapa Geológico de España E. 1:50000. Segunda serie, I.G.M.E., Madrid.
- 11.Rieppel O. 2001. Tooth implantation and replacement in Sauropterygia. Paläont. Z. 75, 207–217. ( 10.1007/BF02988014) [DOI] [Google Scholar]
- 12.Chun L, Rieppel O, Long C, Fraser NC. 2016. The earliest herbivorous marine reptile and its remarkable jaw apparatus. Sci. Adv. 2, e1501659 ( 10.1126/sciadv.1501659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Keefe FR, Otero RA, Soto-Acuña S, O'Gorman JP, Godfrey SJ, Chatterjee S. 2017. Cranial anatomy of Morturneria seymourensis from Antarctica, and the evolution of filter feeding in plesiosaurs of the Austral Late Cretaceous. J. Vertebr. Paleontol. 37, e1347570 ( 10.1080/02724634.2017.1347570) [DOI] [Google Scholar]
- 14.Collin R, Janis CM. 1997. Morphological constraints on tetrapod feeding mechanisms: why were there no suspension feeding marine reptiles? In Ancient marine reptiles (eds Callaway JM, Nicholls EL), pp. 451–466. San Diego, CA: Academic Press. [Google Scholar]
- 15.Motani R, Chen X-H, Jiang D-Y, Cheng L, Tintori A, Rieppel O. 2015. Lunge feeding in early marine reptiles and fast evolution of marine tetrapod feeding guilds. Sci. Rep. 5, 8900 ( 10.1038/srep08900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reif WE, Stein F. 1999. Morphology and function of the dentition of Henodus chelyops (Huene, 1936) (Placodontia, Triassic). Neues Jahrb. Geol. Paläontol. Monatsh. 2, 65–80. [Google Scholar]
- 17.Houssaye A. 2009. ‘Pachyostosis’ in aquatic amniotes: a review. Integr. Zool, 4, 325–340. ( 10.1111/j.1749-4877.2009.00146.x) [DOI] [PubMed] [Google Scholar]
- 18.Houssaye A, Sander PM, Klein N. 2016. Adaptive patterns in aquatic amniote bone microanatomy—more complex than previously thought. Integr. Comp. Biol. 56, 1349–1369. ( 10.1093/icb/icw120) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Figures, data matrix modifications, the data matrix file and information on Simosauridae are available as the electronic supplementary material.