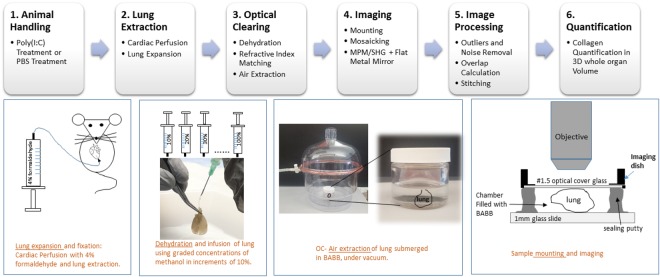
Figure 1.
Whole Lung Optical Clearing and Imaging Workflow. 1. A murine poly(I:C) fibrosis model was used. Animals were treated with intranasal poly(I:C) to induce fibrosis or PBS as a control. 2. Lungs were extracted following cardiac perfusion in ~4 °C with PBS followed by 4% formaldehyde and were expanded post-extraction. 3. Lungs were treated with graded concentrations of methanol (second panel, bottom) for dehydration. Lungs were optically cleared by treatment with BABB for refractive index matching. Air microbubbles were removed by placing lungs in a vacuum chamber (third panel, bottom). 4. Samples were mounted (rightmost bottom panel) in an image chamber between a cover glass and a microscope slide. MPM/SHG mosaic imaging was performed by taking sequential z-stacks through the full depth of the lung (4 × 0.16 N.A. air objective, z interval: 5 µm n = 600–800 planes) across the entire organ with intrinsic tissue auto Fluorescence (MPM) and collagen SHG providing image contrast for the 3D volumetric acquisitions. 5–6. Image stack stitching was done by Fourier Transform Phase Correlation and quantification of collagen deposition (SHG) using 3D image analysis software.