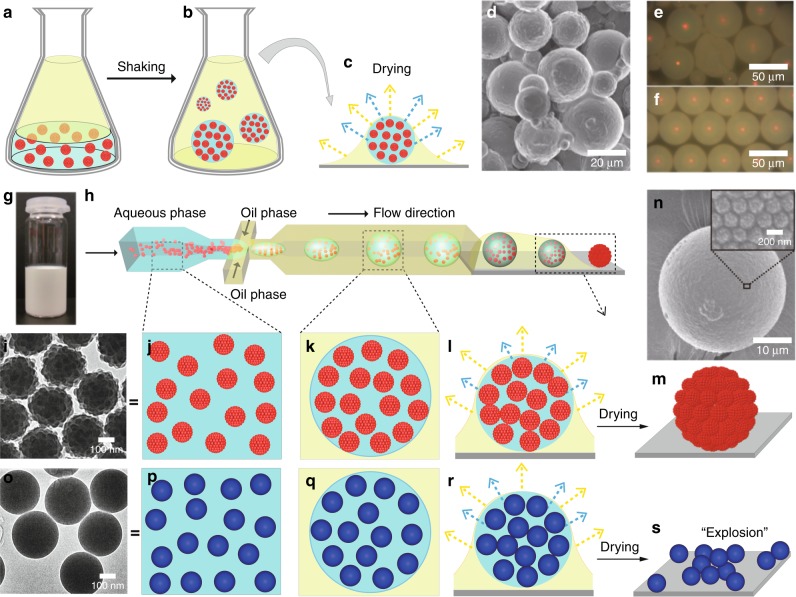
Fig. 3.
Difference in colloidal stability of emulsion droplets made of either raspberry or smooth particles. a A ∼20 w% dispersion of raspberries is mixed in 1:3 parts with fluorinert oil FC40 containing 0.01 w% surfactant FX171 (Sigma Aldrich) (b), vigorously mixed (c) and then left to dry. d SEM image of the resulting dried superspheres. e, f Microscopic images taken in reflection from superspheres prepared by the manual emulsification process and via microfluidics, respectively. g Photograph of the raspberry solution. h Schematic of emulsion-droplet preparation of raspberry dispersions via microfluidics. The dispersion (∼20 w%) is injected (flow rate: 100 μL/h) into a device with a T-junction geometry with the same fluorinert oil and surfactant (flow rate: 200 μL/h) budding off the colloid-containing water droplets. The resulting droplets are then dried in air on a glass substrate. i–m TEM image of the dried raspberries and enlarged schematics of the supersphere-formation process via microfluidics are shown. n SEM images of a supersphere made of raspberry particles and a zoomed-in section, showing the colloidal crystallinity at the surface. o TEM image of similarly sized smooth PS particles. p–r Cartoon showing the emulsion-droplet formation containing the smooth PS particles under the same conditions as for raspberries. s The droplets that were initially stable in oil suddenly burst in the drying process, leaving the PS particles randomly distributed on the substrate (Supplementary Movie 2)