Abstract
Stereotactic body radiotherapy (SBRT) is a form of radiotherapy that delivers high doses of irradiation with high precision in a small number of fractions. However, it has not frequently been performed for the liver due to the risk of radiation-induced liver toxicity. Furthermore, liver SBRT is cumbersome because it requires accurate patient repositioning, target localization, control of breathing-related motion, and confers a toxicity risk to the small bowel. Recently, with the advancement of modern technologies including intensity-modulated RT and image-guided RT, SBRT has been shown to significantly improve local control and survival outcomes for hepatocellular carcinoma (HCC), specifically those unfit for other local therapies. While it can be used as a stand-alone treatment for those patients, it can also be applied either as an alternative or as an adjunct to other HCC therapies (e.g., transarterial chemoembolization, and radiofrequency ablation). SBRT might be an effective and safe bridging therapy for patients awaiting liver transplantation. Furthermore, in recent studies, SBRT has been shown to have a potential role as an immunostimulator, supporting the novel combination strategy of immunoradiotherapy for HCC. In this review, the role of SBRT with some technical issues is discussed. In addition, future implications of SBRT as an immunostimulator are considered.
Keywords: Hepatocellular carcinoma, liver tumors, radiotherapy, stereotactic body radiotherapy, intensity-modulated radiotherapy, immunotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 80–90% of primary liver cancers. Worldwide, it is the fifth most common cancer and the third most common cause of cancer-related death.1 With no specific treatment, the prognosis is very poor, and the median survival for patients with early and advanced tumors is 6–9 months and 1–2 months, respectively.2,3 Several treatment options based on clinical guidelines are available, including surgical resection, percutaneous and transarterial interventions, systemic chemotherapy, liver transplantation (LT), and radiotherapy (RT).
Previously, the role of RT for HCC has been questioned due to the relatively low radiation tolerance of the liver. However, with increasing clinical use and experiences, RT can be considered an effective and safe local therapy for HCC. Moreover, the recent development of modern RT technologies, including image-guided RT (IGRT), intensity-modulated RT (IMRT), and stereotactic body RT (SBRT), has expanded the indications of RT. Compared to three-dimensional conformal RT (3D-CRT), advantages of IMRT include inverse treatment planning method and possible use of many treatment fields, which provide high precision and good conformal dose distribution. Specifically, SBRT can be a useful locoregional treatment option for selected patients who are ineligible for transarterial chemoembolization (TACE) or radiofrequency ablation (RFA) and who have unresectable, small hepatic lesions. Furthermore, SBRT precisely delivers higher ablative doses of irradiation in smaller volumes, providing excellent local control (LC) with potential survival benefits in HCC.
In this review, we will discuss the current role of SBRT for HCC as well as future implications of its use as an immunostimulator.
THE PRINCIPLE OF STEREOTACTIC BODY RADIOTHERAPY
SBRT uses stereotactic non-coplanar conformal RT intended for a small number (usually 1–5 fractions) of significantly larger fraction sizes (usually 8–12 Gy/fraction) while limiting the dose to adjacent normal tissues.4 The sophisticated and steep dose gradient within the target volume usually leads to excellent conformity with steep dose fall-off and high dose delivery to the target volume. Since its development to treat intracranial malignancies, SBRT has been extended to treat a wide variety of extracranial tumors. SBRT for liver tumors was first introduced in the 1990s5; however, it has not frequently been performed because of the risk of radiation-induced liver disease (RILD). Furthermore, liver SBRT is cumbersome because it requires accurate target localization, patient repositioning, control of breathing-related movement, and confers a toxicity risk to the adjacent small bowels.
Over the years, many retrospective studies have been performed with larger cohorts, and several phase I/II trials that enrolled patients who were ineligible for other local therapies have been conducted. Based on the vast body of evidence accumulated as a result of these studies and trials, the recent National Comprehensive Cancer Network guidelines introduced SBRT as an option only in inoperable, ineligible for transplant tumors (category 2B).6 However, currently, technological advances have made it possible for radiation to be precisely delivered to small liver tumors, while further reducing the risk of RILD. The role of SBRT for HCC should be reevaluated further with those recent advances in mind, as previous phase III trials lacked comparisons with other local therapies.
Patient selection
In the currently used guidelines,6,7,8,9 the recommended applications of RT vary (Table 1). Although there is no specific recommendation for SBRT, several guidelines indicate external beam RT (EBRT) as an alternative option for single HCC without vascular invasion. Careful patient selection is required for SBRT in HCC. Although there is no definite limit to the size or number of tumors that can be treated with SBRT, most groups included patients with a maximum lesion size of ≤5 cm, although some included patients with tumors up to 10 cm.10,11 Regarding the number of lesions, patients with 1–3 tumors were usually treated, but again no cut-off value has been established. The number and size of tumors that can be considered for SBRT are dependent on the non-tumoral liver volume that can be spared (usually 700 cm3). SBRT is also feasible for some lesions that are ineligible for surgery or percutaneous ablation. These unique cases require clinical consideration. RFA is not indicated for tumors in the liver dome, given the difficulty of sono-guided tumor targeting under a poor sonographic visual field. Post-RFA failures are also frequent in the S1 region, especially the porta hepatis, because of the high risk of complications or multivascular tumor supply. Similarly, patients who are eligible for SBRT usually have a central lesion of the liver dome, with direct invasion into the vessels, and/or with an insufficient outcome from other local therapies, including RFA and TACE (Fig. 1). Regarding liver function, Child-Pugh class A or upper B are considered appropriate for SBRT, similar to the general guidelines of conventional RT for HCC. Dose modifications and strict dose constraint adherence would be required for tumors with Child-Pugh class B status.12 The safety of liver radiation for HCC in patients with Child-Pugh class C cirrhosis has not been established, as it is unlikely that clinical trials are available for Child-Pugh class C patients.13,14
Table 1. Comparison of Treatment Guidelines for Stereotactic Body Radiotherapy-Eligible Hepatocellular Carcinoma.
| Guidelines | |||||
|---|---|---|---|---|---|
| BCLC | NCCN | APPLE | KLSCG-NCC | ||
| Single, ≤2 cm, without VI | Subgroup | Very early | Resectable or transplantable | Very early | mUICC Stage I |
 |
Primary or preferred option | Resection (or LT/RFA/ PEI, if portal pressure/ bilirubin increased) | Resection or LT | Resection (or LT/RFA/PEI, if portal pressure/bilirubin increased) | Resection or RFA |
| Alternative option | (−) | Locoregional treatment (Ablation, arterial directed therapies, EBRT) | EBRT | TACE, PEI, or EBRT | |
| Single, >2 cm, without VI | Subgroup | Early | Resectable or transplantable | Early | mUICC Stage II |
 |
Primary or preferred option | LT or RFA/PEI | Resection or LT | LT or RFA/PEI | Resection or RFA |
| Alternative option | (−) | Locoregional treatment (Ablation, arterial directed therapies, EBRT) | SABR, hypofractionated RT | TACE, LT, or EBRT | |
BCLC, Barcelona clinic liver cancer; NCCN, National Comprehensive Cancer Network; APPLE, Asia Pacific Primary Liver Cancer Expert Meeting; KLCSG-NCC, Korean Liver Cancer Study Group and the National Cancer Center; VI, vascular invasion; LT, liver transplantation; RFA, radiofrequency ablation; PEI, percutaneous ethanol injection; EBRT, external-beam radiotherapy; mUICC, modified Union for International Cancer Control; TACE, transarterial chemoembolization; RT, radiotherapy; SABR, stereotactic ablative radiotherapy.
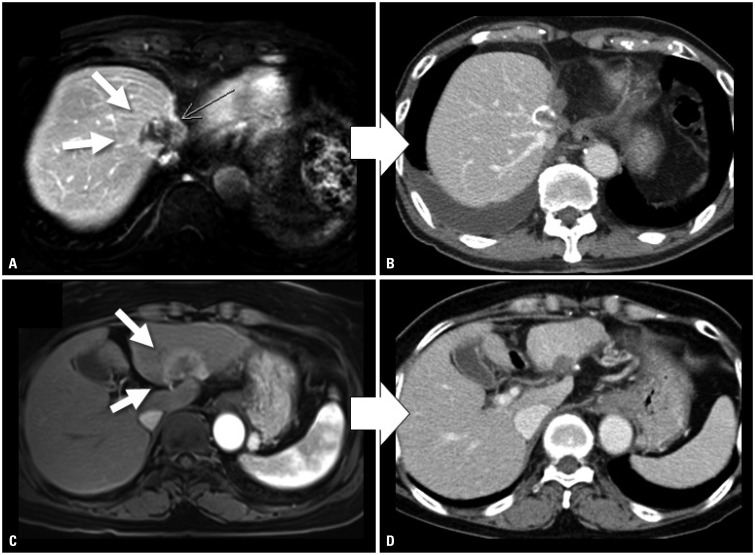
Fig. 1. CT scans of patient cases showing objective responses to SBRT of 60 Gy in 4 fractions for HCC. (A) Before SBRT, the CT scan showed a 3 cm-sized viable HCC after multiple TACE treatments at the dome of the liver (white arrows) (AFP/PIVKA-II: 1017 ng/mL/95 mAU/mL). (B) After SBRT, the 1-year post-SBRT CT scan showed radiologic CR with significantly decreased tumor markers approximating normal levels (AFP/PIVKA-II: 2.04 ng/mL/14 mAU/mL). (C) Before SBRT, a 4 cm-sized HCC was observed in the left lobe (white arrows) (AFP/PIVKA-II: 6.14 ng/mL/31 mAU/mL). (D) After SBRT, the 4-month post-SBRT CT scan showed radiologic CR with further reduced tumor marker levels (AFP/PIVKA-II: 3.38 ng/mL/31 mAU/mL). SBRT, stereotactic-body radiotherapy; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; AFP, alpha-fetoprotein; PIVKA-II, prothrombin-induced by vitamin K absence or antagonist-II; CR, complete response.
Liver toxicity after SBRT
The probability of RILD can increase when the radiation dose to the whole liver exceeds 35 Gy. Owing to advances in technology, partial liver irradiation has been successful in reducing the risk of RILD. According to Dawson and Ten Haken's15 review, if the effective liver volume irradiated is less than 25%, very high RT doses (higher than 100 Gy) may be delivered with low risk of liver toxicity, as long as the liver function is proper. Therefore, SBRT to irradiate only a small volume of the liver can be performed safely for liver tumors. The most common SBRT fractionation schemes for HCC according to the current literature include 3 fractions×10–20 Gy/fx, 4–6 fractions×8–10 Gy/fx, and 10 fractions×5–5.5 Gy/fx.16 Compared with patients with liver metastases, however, those with HCC are more prone to liver toxicity after SBRT, in part because of underlying liver disease and comorbidities.17 In some studies, an increasing Child-Pugh score, a measure of liver toxicity correlating with overall survival (OS), occurred in 10% and 30% of patients with early- and advanced-stage HCC within 3 months after SBRT, respectively.14,18 However, there is a paucity of data correlating the incidence of liver toxicity with SBRT dosimetric parameters for HCC. One Canadian group reported the risk factors associated with a decline in liver function after 6-fraction SBRT for HCC.19 Liver toxicity was defined as an increase in Child-Pugh score (≥2) 3 months after SBRT. Baseline Child-Pugh scores (5 vs. 6) and higher liver doses (e.g., mean dose, effective volume, and doses to 700–900 cc) were strongly associated with liver function decline 3 months after SBRT. A lower baseline platelet count and portal vein thrombus were also associated with an increased risk.
Respiration control
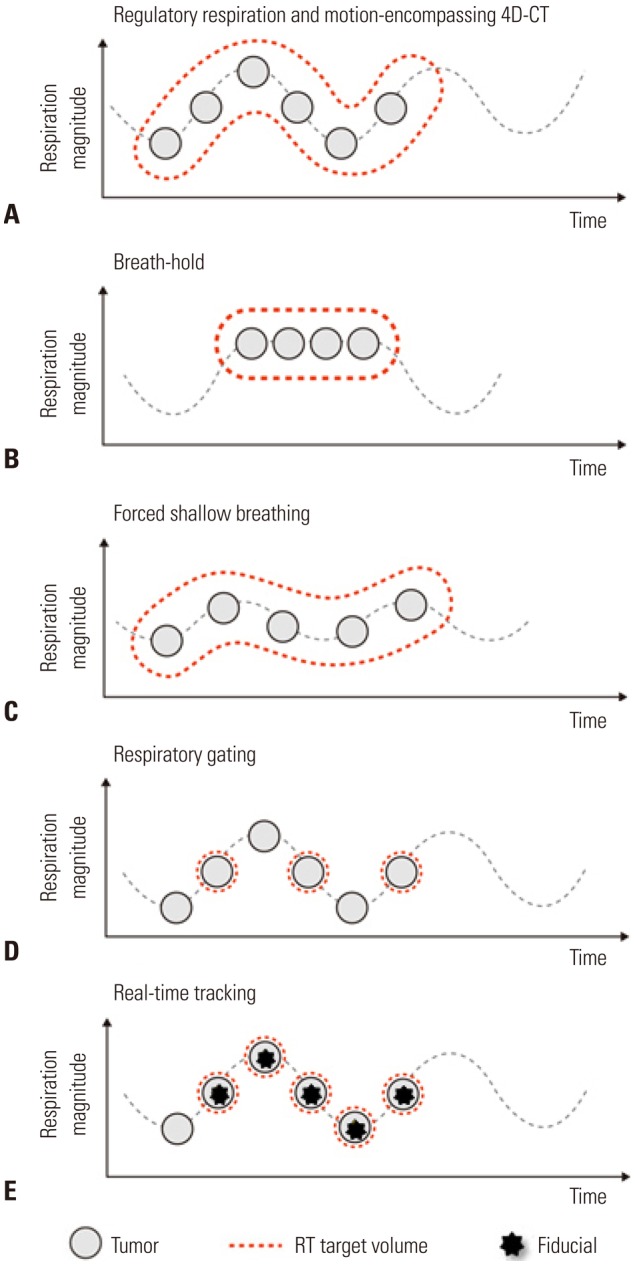
A critical issue in RT for HCC is the control of respiratory movement, as the liver moves over a considerable range during respiration. Several ways may be used to treat a moving tumor to ensure it receives the intended dose while reducing the dose to surrounding normal tissue: motion-encompassing four-dimensional CT (4D-CT) images, breath-hold, forced shallow breathing, respiratory gating, and real-time tumor tracking (Fig. 2). For SBRT, control of respiratory motion should be considered more strictly than for conventional RT. When performing SBRT, many institutions use not only 4D-CT images but also other respiratory control methods. Specifically, real-time tumor tracking using the surrogate on the body surface or an internal fiducial marker has demonstrated high accuracy. Internal fiducial markers can be residual radioopaque lipiodol after TACE or a commercial fiducial marker which is inserted under sono-guidance.
Fig. 2. Different motion management methods in RT. (A) The motion-encompassing method refers to the covering of all possible positions of the moving tumor through the whole breathing cycle using 4D-CT images. Subsequently, a large volume of normal tissue may be irradiated. (B) The breath-hold method refers to let the patient hold breaths for a few seconds under deep inspiration, and then deliver the radiation only when the liver is in a certain position. (C) The forced shallow breathing is a method of using a special external device such as an abdominal compressor to allow the patient to breathe shallow during radiation therapy. Although breath-hold and forced shallow breathing might result in patient discomfort or inconvenience during treatment, it can reduce the respiratory motion for liver tumors and enhance the accuracy. (D) The respiratory gating method is a method of turning on the radiation beam only during a specific respiratory cycle, after accurately grasping the position of a tumor according to a patient's respiratory cycle in advance using 4D-CT images. (E) The real-time tracking method refers to tracking the movement of the tumor along the respiratory cycle using the surrogate on the abdominal surface or internal fiducial marker and then delivering the radiation following the tumor inside the body. No respiratory control and abdominal compression are needed. RT, radiotherapy; 4D, four-dimensional.
Target delineation and dose constraints
Liver SBRT can be performed with various planning machines (e.g., step-and-shoot IMRT, volumetric-modulated arc therapy, tomotherapy, and cyberknife). Several target volumes that are carefully contoured by a professional radiation oncologist are required for SBRT. Gross tumor volume (GTV) is defined as a primary tumor plus an abnormal portal area as indicated by magnetic resonance imaging (MRI). For patients whose lesions are not easily detected by CT, incorporation of MRI in planning may also be necessary for more precise target delineation. The deformable image registration method could be also usefully utilized.20 Clinical target volume (CTV) is defined as GTV plus a 0.3–0.5 cm margin for subclinical disease. Internal target volume is defined as the envelope of all CTVs from the different respiratory phases and is used for treatment planning as the equivalent of the planning target volume for SBRT. In addition to the target volume, the entire liver, the remaining normal liver, both kidneys, the stomach, the duodenum, and the spinal cord should be meticulously contoured and taken into consideration during SBRT planning. In addition to the dose constraint guidelines of the American Association of Physicists in Medicine Task Group,21 the following explicit planning objectives should be defined when executing SBRT plans: 1) ≥700 mL of remaining normal liver should receive ≤15 Gy; 2) maximum dose (Dmax) to bowel, duodenum, and stomach, <24 Gy; 3) Dmax to spinal cord, <18 Gy; and 4) ≥67% of each kidney should receive <15 Gy and V15 should be <35%. According to the 2014 Korean Liver Cancer Study Group guidelines,8 normal liver volume receiving a total dose of <15 Gy must be ≥700 mL, or the mean normal liver dose (liver minus GTV) must be limited to <28 Gy for liver SBRT.
CLINICAL OUTCOME OF STEREOTACTIC BODY RADIOTHERAPY FOR HEPATOCELLULAR CARCINOMA
Curative therapies can improve survival in early-stage HCC patients. Resection, percutaneous ethanol injection, and RFA have been considered effective local treatment options. However, when the effect of these treatments was limited due to the primary tumor location, RT can be considered as an alternative definitive or salvage treatment. Moreover, RT may be a feasible alternative for patients with inoperable tumors or who refuse surgery. In several prospective10,12,13,14,22,23,24,25,26 and retrospective11,27,28,29,30,31,32,33,34,35,36,37 papers, SBRT has been reported to be an effective, safe, and noninvasive treatment modality in newly diagnosed or recurrent cases when the position and size of the tumor are acceptable. In a recent meta-analysis,38 SBRT exhibited an LC rate of 87% with tolerable acute toxicities of grade >3 (4.9%) in HCCs.
Several prospective (phase I or II) trials that reported good outcomes of SBRT in HCCs have been conducted since 2001 (Table 2). In 2010, Goodman, et al.24 conducted a phase I study in patients with HCC or metastatic liver tumors. Radiation doses were escalated from 18–30 Gy in 1 fraction, and the 1-year LC rate was 77% with acceptable toxicity. The 2-year OS rate was 50% at a median follow-up of 17 months. Cardenes, et al.12 and Andolino, et al.13 performed prospective studies using 3–5-fraction SBRT in HCC patients with Child-Pugh class A or B disease. For Child-Pugh class A patients, 36–48 Gy in 3 fractions (dose-escalated) or 44 Gy in 3 fractions were used, while 40 Gy in 5 fractions were used for Child-Pugh class B patients. In all patients, the LC rate was higher than 90%, and the 2-year OS rate was 60%. In 2012, a phase II Korean study evaluated SBRT [median 57 Gy in 3 fractions (range, 42–60 Gy)] in 47 patients with early-stage HCC.10 The 2-year LC and OS rates were 94.6% and 68.7%, respectively. SBRT was well tolerated with a Child-Pugh class increase from A to B only in 13% of patients after RT. In Canada, Bujold, et al.14 reported phase I/II studies of 6-fraction SBRT in 102 Child-Pugh class A patients with advanced HCC who were ineligible for standard locoregional therapies. The median tumor size was 7.2 cm, which was higher than reported by other groups, and 55% of patients had portal vein thrombosis. The prescribed dose was 36 Gy in 6 fractions (range, 24–54 Gy), with the dose based on spared liver volume or other limiting normal tissues. After a median follow-up of 31 months, the 1-year LC rate was 87%, and median survival was 17 months. Outcomes following SBRT for patients with liver dysfunction have also been prospectively assessed in 59 Child-Pugh class A-B9 patients.26 The median volume of HCC was 33.6 cc, and 20% of participants had tumor thrombosis. The median OS of Child-Pugh class A and B patients was 45 and 17 months, respectively, with estimated 2-year LC rates of 92% and 82%, respectively. Four (11%) Child-Pugh class A patients and 8 Child-Pugh class B patients (38%) experienced grade III/IV liver toxicity. For Child-Pugh class B patients (but not A), a dose to remained liver volume was associated with an increased risk of toxicity.
Table 2. Prospective Phase I/II Studies of Stereotactic Body Radiotherapy for HCC.
| Reference | Design | RT Aim | Patient number | Indication | Median size (range), cm | Dose | Median f/u (range), mo | Local control | Overall survival |
|---|---|---|---|---|---|---|---|---|---|
| Goodman, et al.24 | Phase I | Definitive/Salvage | HCC/metastasis (2/24) | CP-A, Unresectable, tumors <5 | Dose escalation 18–30 Gy/1 fx | 17 (2–55) | 77% (1 yr) | 50% (2 yr) | |
| Cardenes, et al.12 | Phase I | Definitive | All HCC (17) | CP-A, CP-B, 1-3 lesions, ≤6 cm, PVT allowed, unavailable for resection | CP-A: 36–48 Gy/3 fx CP-B: 40 Gy/5 fx |
24 (10–42) | 100% | 75% (1 yr) 60% (2 yr) |
|
| Andolino, et al.13 | Phase II | Definitive | All HCC (60) | CP-A, CP-B, liver-confined HCC, prior TACE included | 3.2 (1–6.5) | CP-A: 44 Gy/3 fx CP-B: 40 Gy/5 fx |
27 (2–52) | 90% (2 yr) | 67% (2 yr) |
| Kang, et al.10 | Phase II | Salvage | All HCC (47) | CP-A, CP-B, Inoperable, Incomplete response after TACE, PVT allowed | 2.9 (1.3–7.8) | 57 (42–60) Gy/3 fx | 17 (6–38) | 95% (2 yr) | 69% (2 yr) |
| Bujold, et al.14 | Phase I/II [Trial 1], subsequently phase II [Trial 2] | Definitive | All HCC (102) (trial 1: 50, trial 2: 52) | CP-A, PVT allowed, [trial 2] ≤5 tumors, <15 cm | 7.2 (1.4–23.1) | 36 (24–54) Gy/6 fx | 31 (2–36) | 87% (1 yr) | Median 17 mo |
| Kim, et al.25 | Phase I | Definitive/Salvage | All HCC (18) | CP-A, CP-B, ≤3 tumors, single ≤5 cm, multiple ≤sum 6 cm, previous Tx allowed | 1.9 (1.0–3.3) | Dose escalation 36–60 Gy/4 fx | 23 (11–38) | Radiologic CR: 89%, 49% (2 yr) | 69% (2 yr) |
| Lasley, et al.26 | Phase I/II | Definitive/Salvage | All HCC (59) | CP-A, CP-B, (Phase I → all CP-B possible, Phase II → only CP-B >7 possible) single ≤6 cm, multiple ≤sum 6 cm, | 33.6 (2.0–107.3) cc | CP-A: 38 Gy/3 fx CP-B: 40 Gy/5 fx |
33 (3–61) | 92% for CP-A and 82% for CP-B (2 yr) | Median 45 mo for CP-A, 17 mo for CP-B; 2-yr 72% for CP-A, 33% for CP-B (p=0.03) |
RT, radiotherapy; HCC, hepatocellular carcinoma; CP, Child-Pugh; PVT, portal vein thrombosis; TACE, transarterial chemoembolization; CR, complete response.
Multiple retrospective studies have also reported excellent outcomes with SBRT for patients with HCCs (Table 3). Published results of several retrospective studies have largely included instances in which surgical resection or percutaneous ablative therapies were difficult, unfeasible, or rejected, as well as some pools of intermediate or advanced-stage HCC. Overall, promising outcomes with LC rates of 64–95% and OS rates of 43–87% at 2 years were reported. The wide variation in OS is due in part to different patient characteristics and the presence of tumor thrombosis in some series.
Table 3. Recent Retrospective Studies of SBRT for HCC.
| Reference | Design | RT Aim | Patient number | Indication | Median size (cm) | Dose | Median f/u (range), mo | Local control | Overall survival |
|---|---|---|---|---|---|---|---|---|---|
| Kwon, et al.28 | Cyberknife | Definitive/Salvage | 42 | CP-A, CP-B, ≤100 cc, unavailable for other local therapies, no EHM | 30–39 Gy/3 fx | 29 (8–49) | 72% (1 yr), 68% (3 yr) | 93% (1 yr), 59% (3 yr) | |
| Seo, at al.11 | Cyberknife | Salvage | 38 | CP-A, CP-B, <10 cm, all with TACE failure | 33–57 Gy/3–4 fx | 15 (3–27) | 66% (2 yr) | 61% (2 yr) | |
| Louis, et al.29 | Cyberknife | Definitive/Salvage | 25 | CP-A, CP-B, PVT allowed, previous TACE, op, RFA, nexavar included | 4.5 (1.8–10) | 45 Gy/3 fx | 13 (1–24) | 95% (1 yr) | 79% (1 yr) |
| Huang, et al.30 | Cyberknife, Matched pair analysis (SBRT vs. other/no Tx) | Salvage | 36 | CP-A, CP-B, CP-C, all with prior Tx, but tumor progressed | 4.4 (1.1–12.3) | 37 (25–48 Gy)/4–5 fx | 14 (2–35) | 88% (1 yr), 75% (2 yr) | 64% (2 yr) |
| Honda, et al.32 | Linac, TACE alone vs. TACE → SBRT | Salvage | 30 | CP-A, CP-B, solitary, ≤3 cm, all prior TACE, no PVT, no EHM | 1.6 (1–3) | 48 Gy/4 fx or 60 Gy/8 fx | 12 (6–38) | 100% | 100% (1 yr), 100% (3 yr) |
| Jang, et al.33 | Cyberknife | Definitive/Salvage | 108 | CP-A, CP-B, unsuitable for other Tx or incomplete TACE | 3 (1–7) | 51 (33–60)/3 fx | 30 (4–81) | 87% (2 yr) | 63% (2 yr) |
| Sanuki, et al.31 | Linac | Definitive/Salvage | 185 | CP-A, CP-B, single, unresectable, no LN mets or EHM | 2.7 (1–5) | CP-A: 40 Gy/5 fx CP-B: 35 Gy/5 fx |
24 (3–80) | 91% (3 yr) | 70% (3 yr) |
| Culleton, et al.34 | 3D-CRT, IMRT, or VMAT | Definitive/Salvage | 29 | CP-B, <10 cm, <5 tumors, life expectancy >3 months, KPS >60%, unresectable | 8.66 (4.1–26.6) | Median 30 Gy/6 fx | 12 SD, 2 PR 6 intrahepatic PD (outfield) | Median 8 months, 32% (1 yr) | |
| BCLC-C | |||||||||
| Bae, et al.35 | Cyberknife | Definitive/Palliative | 35 | BCLC-C, CP-A, CP-B, vascular invasion or EHM | 45 (30–60) Gy /3–5 fx | 14 (1–44) | 69% (1 yr), 51% (3 yr) | 52% (1 yr), 21% (2 yr) | |
| Huge HCC | |||||||||
| Que, et al.36 | Cyberknife | Definitive/Salvage | 22 | CP-A, CP-B, ≥10 cm, ECOG≤2 | 11.36 (10–18) | Mainly 40 Gy/5 fx (26–40) | 11.5 (2–46) | 56% (1 yr), response rate 86.3% | Median 11 mo, 50% (1 yr) |
| Zhong, et al.37 | Total body gammaray stereotactic RT system | Salvage | 72 | Incomplete TACE → SBRT, ≥10 cm, ECOG≤2 | 12.6 (10.8–16.5) | 35.7 (33.8–39)/6 fx | 18 (4–70) | Response rate 79%, low incidence of recur (8%) | Median 11 mo, 38% (1 yr), 12% (3 yr), 3% (5 yr), significantly higher with tumor encapsulation (56%, 1yr) |
| Kuo, et al.41 | Cyberknife | Definitive/Salvage | ≤4 cm: 52, >4 to <10 cm: 355, ≥10 cm: 34 CP-A, CP-B≤7 | 1.8–18 | 26–40 Gy /3–5 fx for tumors >5 cm, 39 Gy/3 fx for tumors ≤5 cm | 16 (2–72) | Response rate 96%/91%/76% (p<0.001) | 50%, 45%, 33% (3 yr) (no significant difference by size) | |
RT, radiotherapy; HCC, hepatocellular carcinoma; CP, Child-Pugh; EHM, extrahepatic metastasis; TACE, transarterial chemoembolization; PVT, portal vein thrombosis; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; VMAT, volumetric modulated arc therapy; LN, lymph node; KPS, Karnofsky performance status; SD, stable disease; PR, partial response; PD, progressive disease; BCLC, Barcelona-Clinic Liver Cancer.
Huang, et al.30 performed Cox multivariable regression hazard analysis to compare OS curves of SBRT (n=36) and non-SBRT (n=138) patient groups [hazard ratio (HR), 2.44; p=0.005] after adjustment for possible prognostic factors. The 2-year OS rates of SBRT recipients (n=28) and matched controls (n=28) (72.6% vs. 42.1%; p=0.013) were compared, concluding that SBRT significantly improved survival in patients with recurrent unresectable HCC. Honda, et al.32 showed that SBRT combined with TACE is an effective and safe locoregional treatment of small solitary primary HCC with a 96% complete response (CR) rate. In the largest series, Sanuki, et al.31 reported on the outcomes of 185 patients with solitary HCCs ≤5 cm in size that were unsuitable for surgery or RFA. A total dose of 40 Gy or 35 Gy in 5 fractions was prescribed for Child-Pugh class A and B patients, respectively. Three-year LC and OS rates were 91% and 70%, respectively, with no significant difference between dose levels. However, acute toxicity of ≥grade 3 was observed in 13% of patients, occurring more frequently in Child-Pugh class B patients. Occasionally, SBRT was also used for tumor thrombosis in portal vein or inferior vena cava,39 Barcelona-Clinic Liver Cancer stage C HCC,35 Child-Pugh class C patients,40 or large HCC ≥10 cm36,37,41 with acceptable response rates and survival outcomes. Jang, et al.33 suggested that there was a positive linear relationship between SBRT dose and 2-year LC and OS rates, which were highest at >54 Gy. As several clinical trials have demonstrated the efficacy and safety of SBRT, modern SBRT could provide an ablative dose to a tumor while sparing the remaining liver parenchyma.
SBRT as a curative treatment comparable to RFA
According to the current guidelines of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases,42,43 RFA is recommended as the first-line treatment for early-stage HCC. However, RFA has significant limitations, including size discrepancies, tumor proximity to major vascular or biliary structures, and limited accessibility on ultrasonography.44 Since RFA is based on the principle of frictional heat production by frequency waves, heat conduction rates decrease with increasing tumor size, resulting in inadequate tumor control. It is also well accepted that RFA of tumors in the liver dome is limited due to the invisibility under ultrasonography guidance. Several reports have described that either subphrenic location or a short distance from the diaphragm is associated with higher local recurrence after RFA.45,46 Another study showed that approximately half of the tumors located in the liver dome recurred within 3 years after RFA due to inadequate ablation resulting from difficulties visualizing the tumor. SBRT can be a useful alternative in these situations. SBRT has a less strict indication for the size of tumors as discussed below. Furthermore, the therapeutic approach is not limited by tumor location when SBRT is implemented with daily IGRT. However, until now, little evidence has been available to determine the efficacy of SBRT compared to RFA.
Recently, a noteworthy study has attracted widespread attention from physicians due to the potential applications of SBRT and RFA. Wahl, et al.47 compared the outcomes of patients with nonmetastatic, inoperable HCCs who received SBRT or RFA. For tumors <2 cm, there was no significant difference between two treatments in freedom from local progression (FFLP) [HR, 2.50; 95% confidence interval (CI), 0.72 to 8.67; p=0.15], but for tumors ≥2 cm, RFA was associated with a significantly worse FFLP (HR, 3.35; 95% CI, 1.17 to 9.62, p=0.025). More recently, an observational study analyzed a large cohort from the National Cancer Database to compare the effectiveness of RFA (n=3684) and SBRT (n=296) in patients with stage I or II HCC.48 This study demonstrated the opposite result. The 5-year OS rate was significantly higher in the RFA group [29.8% (95% CI, 24.5–35.3%)] than in the SBRT group [19.3% (95% CI, 13.5–25.9%)] (p<0.001) in both propensity matching and probability-weighted analyses. The benefit of RFA was consistent across all subgroups and was robust to the effects of severe fibrosis or cirrhosis. However, this study may be limited by the biases related to its retrospective design although no other study with a similarly large cohort exists. A randomized clinical trial or more constructive studies would be helpful to confirm the results of these studies. A concerted effort should be made to identify a patient subgroup more suitable for RFA or SBRT shortly.
SBRT as a bridging treatment before liver transplantation
SBRT is also a suitable bridging therapy for patients with HCC awaiting LT. SBRT may enable patients with HCC to remain eligible for curative transplantation pending organ availability.49,50 O'Connor, et al.50 demonstrated that successful orthotopic LT was undertaken following SBRT (median 6.3 months) in 10 patients with 11 HCCs. After the median follow-up of 62 months, the median time from SBRT to LT was 113 days (range, 8–794 days). The 5-year OS and disease-free survival rates were both 100%, with only 4 of 10 patients experiencing acute toxicity. Explant pathology revealed a CR rate of 27% while the remaining 8 tumors decreased or remained stable in size. In a recent paper by Sapisochin, et al.,51 379 patients who received LT after RFA (n=244), SBRT (n=36), or TACE (n=99) were compared to evaluate the efficacy and safety of bridging treatments. The drop-out and postoperative complication rates were similar between the groups. The 3-year survival from the time of transplant was 81% in the RFA group, 75% in the SBRT group, and 75% in the TACE group, which was not significantly different. Theirs was the first study to show that SBRT may be as effective and safe as TACE or RFA when used to maintain the candidacy of patients with HCC on the transplant waiting list.
A NEW ROLE FOR STEREOTACTIC BODY RADIOTHERAPY AS AN IMMUNOSTIMULATOR
Immunotherapy and HCC
Recent progress in the understanding of tumor biology and immune checkpoint has provided novel therapeutic strategies using immune checkpoint blockades. Over the years, different immunotherapeutic approaches have been evaluated for the treatment of HCC. Unlike certain other malignancies, HCC cells do not appear to be inherently immunogenic. However, recent papers that studied checkpoint inhibitors for HCC demonstrated promising results,52,53 and more phase I and II clinical studies are underway to investigate the effects of various immune checkpoint inhibitors, including cytotoxic T-lymphocyte-associated protein-4 inhibitors and anti-programmed death-1 (anti-PD-1) antibody. Nevertheless, modest response rates have indicated that the efficacy of these checkpoint inhibitors appears to be insufficient.54,55 Therefore, the development of combination therapies appears to be necessary, which can not only provide direct tumoricidal effects but also enhance the antitumor effect of immunotherapy.
Recent preclinical studies had suggested that the effects of RT may be enhanced through the dissemination and activation of tumor-associated antigens when RT was delivered in combination with immunotherapeutic agents, especially in a high dose per fraction. One important mechanism that can explain this enhancement is the abscopal effect which means the treatment may affect even untreated areas.56 This strategy is particularly appealing, as conforming RT to a small target volume may enhance the efficacy of immunotherapy in HCC, leading to the eradication of untreated macroscopic lesions and/or occult microscopic disease. Consequently, RT elicited systemic anti-tumor effects mediated by the immune system. Preclinical data using human HCC tumor cultures and murine models demonstrated that RT increased cell surface expression of immunogenicity markers and increased sensitivity to dendritic cell therapy.57,58,59 Although further research is warranted, several recent studies of the combination of RT and immunotherapy in the management of HCC have shown promising results.60,61,62
SBRT as an immunostimulator
Many preclinical studies demonstrated that high-dose per fraction RT has a beneficial outcome over classical RT fractionation,63,64,65 even though some in vitro models suggested an advantage for classical fractionated RT.66,67,68 To identify the specific mechanism and confirm this hypothesis, Kim, et al.69 published a preclinical study. Using the murine HCC cell line HCA-1, the effect of radiation (10 Gy per one fraction) on programmed death-ligand 1 (PD-L1) expression was measured. The signaling pathways involved in the altered PD-L1 expression, tumor growth, and survival rates were also evaluated. Radiation upregulated PD-L1 expression in tumor cells through interferon (IFN)-γ/signal transducer and activator of transcription 3 (STAT3) signaling, which could facilitate the therapeutic action of anti-PD-L1 in the murine HCC model. Furthermore, the combination of anti-PD-L1 and radiation significantly delayed tumor growth compared to treatment with anti-PD-L1 or radiation alone, enhancing survival significantly (7-week survival; 90% vs. 0% or 30%, respectively, p<0.001). The underlying mechanism involved increasing apoptosis, decreasing tumor cell proliferation, as well as the restoration of CD8+ T cell functions.
The same group published another clinical study.70 The soluble PD-L1 (sPD-L1) level was measured (before/after RT, and 1 month after RT) and compared between the SBRT group and the conventional RT group. The sPD-L1 level increased after RT but decreased after 1 month in patients who underwent conventional RT, while it tended to increase continuously for 1 month in SBRT patients. These results suggest that SBRT may be superior to conventional fractionated RT for combined use with immune checkpoint inhibitors. Other well-designed studies of RT combined with immune checkpoint inhibitors would be helpful to clarify the optimal RT scheme, dose, and time to implement the combination.
SBRT has been considered more immunogenic than conventional fractionation.71,72 According to previous studies, several possible mechanisms that act differently from those of conventional RT doses exist. First, although radiation-induced cell death is known to be caused predominantly by radiation-induced DNA damage or breaks, radiation delivered at higher doses per fraction (8–10 Gy) may induce other more significant vascular, stromal, and antitumor immune responses within the local tumor environment.73 Second, there is no danger signal when tumors are untreated, producing limited or tolerogenic antigen presentation. In contrast, upon receiving a tumoricidal dose of radiation, dying tumor cells release a large number of tumor antigens, and those antigens act as danger signals which actively induce the immunogenic response. These tumor antigens also induce maturation of dendritic cells and increase antigen presentation by these cells. These signals result in the activation and proliferation of tumor-specific CD8 T cells. The release of danger signals from tumor cells appears to be more active by ablative dose per 1 fraction than conventional dose per fraction. Third, radiation causes tumor cells to be more susceptible to immunogenic cell death. Radiation upregulates the major histocompatibility complex and first apoptosis signal receptor on tumor cells, increasing their susceptibility to T cell-mediated cytotoxicity as well as increasing PD-L1 on tumor cells.74 Therefore, radiation combined with immune checkpoint inhibitors offer a more effective combination strategy than radiation alone.
CONCLUSION
The use of SBRT for HCC appears to be a useful noninvasive option with an LC rate of approximately 90%. Numerous advances in EBRT allow for more accurate targeting and sparing of normal liver parenchyma, making aggressive dose-fractionation strategies possible in daily practice. SBRT for HCC may be a curative treatment comparable to RFA as well as an effective bridging treatment before LT, although further multi-institutional prospective studies are warranted to confirm these results in the clinical setting. Moreover, a new role for SBRT as an immunostimulator is suggested by enhancing the antitumor effect of immune checkpoint inhibitors in addition to its tumoricidal effect.
ACKNOWLEDGEMENTS
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the illustrations.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Potters L, Steinberg M, Rose C, Timmerman R, Ryu S, Hevezi JM, et al. American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60:1026–1032. doi: 10.1016/j.ijrobp.2004.07.701. [DOI] [PubMed] [Google Scholar]
- 5.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15:563–573. doi: 10.6004/jnccn.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HC, Yu JI, Cheng JC, Zeng ZC, Hong JH, Wang ML, et al. Consensus for radiotherapy in hepatocellular carcinoma from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014): current practice and future clinical trials. Liver Cancer. 2016;5:162–174. doi: 10.1159/000367766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea Practice Guideline for the management of hepatocellular carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 10.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 11.Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 12.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 13.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 15.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang PM, Chung NN, Hsu WC, Chang FL, Jang CJ, Scorsetti M. Stereotactic body radiation therapy in hepatocellular carcinoma: optimal treatment strategies based on liver segmentation and functional hepatic reserve. Rep Pract Oncol Radiother. 2015;20:417–424. doi: 10.1016/j.rpor.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae SH, Kim MS, Jang WI, Cho CK, Yoo HJ, Kim KB, et al. Low hepatic toxicity in primary and metastatic liver cancers after stereotactic ablative radiotherapy using 3 fractions. J Korean Med Sci. 2015;30:1055–1061. doi: 10.3346/jkms.2015.30.8.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660–664. doi: 10.1002/hep.1840070408. [DOI] [PubMed] [Google Scholar]
- 19.Velec M, Haddad CR, Craig T, Wang L, Lindsay P, Brierley J, et al. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2017;97:939–946. doi: 10.1016/j.ijrobp.2017.01.221. [DOI] [PubMed] [Google Scholar]
- 20.Lee DS, Woo JY, Kim JW, Seong J. Re-irradiation of hepatocellular carcinoma: clinical applicability of deformable image registration. Yonsei Med J. 2016;57:41–49. doi: 10.3349/ymj.2016.57.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 22.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 23.Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, et al. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 24.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Seong J, Lee IJ, Woo JY, Han KH. Phase I dose escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget. 2016;7:40756–40766. doi: 10.18632/oncotarget.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, et al. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e443–e449. doi: 10.1016/j.prro.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, et al. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351. doi: 10.1186/1471-2407-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9:479–487. doi: 10.1177/153303461000900506. [DOI] [PubMed] [Google Scholar]
- 30.Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 31.Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 32.Honda Y, Kimura T, Aikata H, Kobayashi T, Fukuhara T, Masaki K, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530–536. doi: 10.1111/jgh.12087. [DOI] [PubMed] [Google Scholar]
- 33.Jang WI, Kim MS, Bae SH, Cho CK, Yoo HJ, Seo YS, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi: 10.1186/1748-717X-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412–417. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Bae SH, Kim MS, Cho CK, Kim KB, Lee DH, Han CJ, et al. Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma. J Korean Med Sci. 2013;28:213–219. doi: 10.3346/jkms.2013.28.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Que JY, Lin LC, Lin KL, Lin CH, Lin YW, Yang CC. The efficacy of stereotactic body radiation therapy on huge hepatocellular carcinoma unsuitable for other local modalities. Radiat Oncol. 2014;9:120. doi: 10.1186/1748-717X-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong NB, Lv GM, Chen ZH. Stereotactic body radiotherapy combined with transarterial chemoembolization for huge (≥10 cm) hepatocellular carcinomas: a clinical study. Mol Clin Oncol. 2014;2:839–844. doi: 10.3892/mco.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2015;114:289–295. doi: 10.1016/j.radonc.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 39.Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabavizadeh N, Waller JG, Fain R, 3rd, Chen Y, Degnin CR, Elliott DA, et al. Safety and efficacy of accelerated hypofractionation and stereotactic body radiation therapy for hepatocellular carcinoma patients with varying degrees of hepatic impairment. Int J Radiat Oncol Biol Phys. 2018;100:577–585. doi: 10.1016/j.ijrobp.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Kuo HT, Que J, Lin LC, Yang CC, Koay LB, Lin CH. Impact of tumor size on outcome after stereotactic body radiation therapy for inoperable hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e9249. doi: 10.1097/MD.0000000000009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 43.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29:558–568. doi: 10.3109/02656736.2013.821528. [DOI] [PubMed] [Google Scholar]
- 45.Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–981. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 46.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as firstline therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the National Cancer Database. J Clin Oncol. 2018;36:600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 49.Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83:895–900. doi: 10.1016/j.ijrobp.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949–954. doi: 10.1002/lt.23439. [DOI] [PubMed] [Google Scholar]
- 51.Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Rai V, Abdo J, Alsuwaidan AN, Agrawal S, Sharma P, Agrawal DK. Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma. Mol Cell Biochem. 2018;437:13–36. doi: 10.1007/s11010-017-3092-z. [DOI] [PubMed] [Google Scholar]
- 53.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mc-Dermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Lin CC, Wang TE, Liu CY, Lin CP, Liu TP, Chen MJ, et al. Potentiation of the immunotherapeutic effect of autologous dendritic cells by pretreating hepatocellular carcinoma with low-dose radiation. Clin Invest Med. 2008;31:E150–E159. doi: 10.25011/cim.v31i3.3472. [DOI] [PubMed] [Google Scholar]
- 58.Kawashita Y, Deb NJ, Garg M, Kabarriti R, Alfieri A, Takahashi M, et al. An autologous in situ tumor vaccination approach for hepatocellular carcinoma. 1. Flt3 ligand gene transfer increases antitumor effects of a radio-inducible suicide gene therapy in an ectopic tumor model. Radiat Res. 2014;182:191–200. doi: 10.1667/RR13594.1. [DOI] [PubMed] [Google Scholar]
- 59.Kawashita Y, Deb NJ, Garg MK, Kabarriti R, Fan Z, Alfieri AA, et al. An autologous in situ tumor vaccination approach for hepatocellular carcinoma. 2. Tumor-specific immunity and cure after radio-inducible suicide gene therapy and systemic CD40-ligand and Flt3-ligand gene therapy in an orthotopic tumor model. Radiat Res. 2014;182:201–210. doi: 10.1667/RR13617.1. [DOI] [PubMed] [Google Scholar]
- 60.Neve Polimeno M, Ierano C, D'Alterio C, Simona Losito N, Napolitano M, Portella L, et al. CXCR4 expression affects overall survival of HCC patients whereas CXCR7 expression does not. Cell Mol Immunol. 2015;12:474–482. doi: 10.1038/cmi.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trautmann F, Cojoc M, Kurth I, Melin N, Bouchez LC, Dubrovska A, et al. CXCR4 as biomarker for radioresistant cancer stem cells. Int J Radiat Biol. 2014;90:687–699. doi: 10.3109/09553002.2014.906766. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61:1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, Barbes B, Solorzano JL, Perez-Gracia JL, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 2016;76:5994–6005. doi: 10.1158/0008-5472.CAN-16-0549. [DOI] [PubMed] [Google Scholar]
- 64.Dewan MZ, Vanpouille-Box C, Kawashima N, DiNapoli S, Babb JS, Formenti SC, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res. 2012;18:6668–6678. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubner Y, Muth C, Strnad A, Derer A, Sieber R, Buslei R, et al. Fractionated radiotherapy is the main stimulus for the induction of cell death and of Hsp70 release of p53 mutated glioblastoma cell lines. Radiat Oncol. 2014;9:89. doi: 10.1186/1748-717X-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kulzer L, Rubner Y, Deloch L, Allgäuer A, Frey B, Fietkau R, et al. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells. J Immunotoxicol. 2014;11:328–336. doi: 10.3109/1547691X.2014.880533. [DOI] [PubMed] [Google Scholar]
- 68.Tsai MH, Cook JA, Chandramouli GV, DeGraff W, Yan H, Zhao S, et al. Gene expression profiling of breast, prostate, and glioma cells following single versus fractionated doses of radiation. Cancer Res. 2007;67:3845–3852. doi: 10.1158/0008-5472.CAN-06-4250. [DOI] [PubMed] [Google Scholar]
- 69.Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget. 2017;8:41242–41255. doi: 10.18632/oncotarget.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol. 2018 Jan 20; doi: 10.1016/j.radonc.2017.11.027. [Epub] [DOI] [PubMed] [Google Scholar]
- 71.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song CW, Lee YJ, Griffin RJ, Park I, Koonce NA, Hui S, et al. Indirect tumor cell death after high-dose hypofractionated irradiation: implications for stereotactic body radiation therapy and stereotactic radiation surgery. Int J Radiat Oncol Biol Phys. 2015;93:166–172. doi: 10.1016/j.ijrobp.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]