Abstract
Aim: Fructus aurantii (FA) is widely used in clinic as an expectorant and digestant herb in traditional Chinese medicine and proven to have a variety of pharmacological functions. FA is close to grapefruit either by botanical taxonomy or by their same components (flavonoids, etc.) and grapefruit has been proven to cause drug–drug interaction when co-administrated with CYP3A4 substrates. Besides, FA contains many compounds, such as flavonoids, which have been reported to impact the expressions of CYP450. However, the effect of FA on CYP450, whose change may affect drug safety and clinical efficacy attributed to drug–drug interaction, still remains unknown.
Methods: The protein, mRNA expression and enzyme activity of CYP1A2, CYP3A4, and CYP2E1 in rat were determined by Western Blotting, RT-PCR method, the cocktail method, respectively, after orally administration of FA in succession for 7 days. CYP1A2, CYP3A4, and CYP2E1 mRNA expression were investigated in HepG2 cells following FA-medicated serum incubation for 24 h.
Results: In rat, compared to the control group, CYP1A2, CYP3A4 protein, and mRNA expression were significantly induced consistent with the corresponding CYP activities; the protein expression of CYP2E1 was significantly upregulated, while the corresponding mRNA expression and enzyme activity showed no significant change. In HepG2 cells, compared to the control group, the mRNA expression of CYP1A2 and CYP3A4 was up-regulated statistically while CYP2E1 mRNA expression was not significantly induced or inhibited.
Conclusion: FA may be a potential slight inducer of CYP1A2 and CYP3A4 and is unlikely to impact CYP2E1 until clinical researches are conducted.
Keywords: Fructus aurantii, CYP1A2, CYP3A4, CYP2E1, drug–drug interaction
Introduction
Fructus aurantii (FA, Zhiqiao in Chinese), the dried and immature fruit of Citrus aurantium L. (Rutaceae) or its cultivars gathered from July, is used as an expectorant and digestant herb in traditional Chinese medicine, which is cited in Chinese Pharmacopoeia (1 part) of 2015 version. It is widely used in clinic as one component of several formulae, such as Xue-Fu-Zhu-Yu Tang (Liu et al., 2004), decoction of Bulpleurum falcatum L. and FA (Li et al., 2004), the formulae of FA and Magnolia bark which has produced relatively favorable effect to functional dyspepsia (Huang et al., 2011; Xiong et al., 2015), Chaihu-Shugan-San (Fan et al., 2012), and Wei-Chang-An-Wan (Liu et al., 2013). FA extract is reported to have a wide range of potential therapeutic effects, such as hypocholesterolemic action (Kim et al., 2011), anti-tumor action (Zhou et al., 2007), prokinetic action (Huang et al., 2011; Zhang et al., 2012), anti-ischemic action (Kang et al., 2007), and anti-depressive action (Fan et al., 2012; Zhang et al., 2012). It has been reported that FA reduces portal pressure in portal hypertensive rats (Huang et al., 1995) and has been frequently used to treat cardiovascular symptoms in Asian countries (Kang et al., 2007; Kim et al., 2011).
The Cytochrome P450 enzymes (CYP450), monooxygenases metabolizing xenobiotics and endogenous substrates, are involved in approximately 80% of oxidative drug metabolism and account for almost 50% of the overall elimination of commonly used drugs (Wilkinson, 2005). Among the Cytochrome P450 enzymes, families 1–3 constitute almost half of total CYPs in mammals (Bièche et al., 2007; Tydén et al., 2014), and CYP1A2, CYP2E1, and CYP3A4 were mainly expressed in the liver (Bièche et al., 2007), which is the major site of Cytochrome P450 mediated metabolism (Wilkinson, 2005). CYP450 was reported to mediate drug–drug interaction. The well-known example is that grapefruit juice increases the bioavailability of various drugs due to the inhibition of CYP3A4 metabolism (Yoshida et al., 2005).
The FA is confirmed to contain flavonoids, furanocoumarins, essential oil, alkaloids, coumarins, and microelement. These compounds are reported to interact with CYPs in many literatures. It is known that flavonoids, considered to be the major bioactive constituents of FA (Tong et al., 2012), can either inhibit or activate human Cytochrome P450 depending upon their structures, concentrations, and experimental conditions (Hodek et al., 2002). Furanocoumarins in Citrus crude drugs is found to inhibit not only CYP3A activity but other CYP subtypes, which may cause in vivo drug interactions (Guo et al., 2001). Thus, we wonder whether FA is potential to exert influence on CYPs and cause drug–drug interaction for reasons as follow: on one hand, FA contains the compounds which were demonstrated to effect CYPs expressions; on the other hand, FA is close to grapefruit either by botanical taxonomy or by their same components (flavonoids, furanocoumarins, etc.) and grapefruit has been proven to influence CYP3A4 contributing to drug–drug interaction.
However, reports on the FA related to influence on CYPs were rarely found. Considering the extensive clinical application of FA and the poor information of the impact of FA on CYPs, it is beneficial that a study, whether FA has effects on CYPs, can be carried out. In this paper, hepatic CYP1A2, CYP2E1, CYP3A4 protein expression, mRNA expression, and enzyme activity were detected in rat after oral administration of FA extract by Western blot, RT-PCR, and cocktail method, respectively. The cocktail method adopted probes including caffeine (CYP1A2), dapsone (CYP3A4) and chlorzoxazone (CYP2E1). Their mRNA expression in HepG2 cells was analyzed by RT-PCR based on HepG2 cell system and serum pharmacological method. For the sake of safety profiles and drug efficacy, this study is worth conducting.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum and 0.25% Trypsin-EDTA were purchased from Gibco Life TechnologiesTM. The 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) were obtained from Sigma–Aldrich Co. Anti-rabbit horseradish peroxidase-conjugated secondary antibody, anti-mouse horseradish peroxidase-conjugated secondary antibody, anti-β-actin antibody, anti-Cytochrome P2E1 antibody, and anti-CYP3A4 antibody were obtained from Abcam. Anti-CYP1A2 rabbit polyclonal antibody was purchased from Sangon Biotech (Shanghai) Co., Ltd. PierceTM BCA Protein Assay Kit and RevertAid First Strand cDNA Synthesis Kit were purchased from Thermo Fisher Scientific. All-in-OneTM qPCR Mix Kit was purchased from GeneCopoeia. TRIzoL Reagent was bought from Invitrogen. Western blotting luminal reagent was obtained from Santa Cruz Biotechnology, Inc. Acetonitrile, methanol, formic acid, and reference substances (caffeine, chlorzoxazone, dapsone, naringenin, hesperetin, and meranzin hydrate) were HPLC grade, other reagents were analytical grade.
Plant Material and Preparation of Fructus aurantii Extract
The herb FA was purchased from the pharmacy of Xiangya Hospital of Central South University, Changsha, China, and authenticated. The voucher specimen (No. 2014070802, Jiangxi, China) was deposited in the Laboratory of Ethnopharmacology, Xiangya Hospital of Central South University. The procedures of extraction were simply described as follow: Herb FA material was thoroughly decocted twice in water (1:8 and 1:6, w/v, respectively) for 30 min at 100°C after soaking for 30 min. The decoction in both times were collected, blended, and filtered through eight layers of gauze twice before concentration with a vacuum rotary evaporator at 56°C. The concentrations of the water extracts we obtain were 0.625, 1.25, and 1.875 of raw material per ml, respectively. The final FA extracts were stored at -20°C prior to use.
Animals
Male Sprague–Dawley rats, weighing 200 ± 20 g, were supplied by Animal Experimental Center in Kaifu District (Changsha, China). This study was strictly implemented according to the Regulations for the Administration of Affairs Concerning Experimental Animals (1988), which were approved by the Animal Experimental Center for Central South University (Changsha, China). The rats were caged (3 rats per standard cage) with free access to food and tap water under strictly controlled conditions (room temperature: 25 ± 2°C, relative humidity: 50 ± 10%, 12 h light/dark cycle). The animals were allowed to adapt to the new environment for 3 days before the commencement of this experiment.
Effect of FA on the CYPs Protein and mRNA Expression in Rats
Treatment With Animals and Preparation of Liver Tissues and FA-Containing Serum Samples
The rats were randomly distributed into four subgroups (six each) and then orally administrated saline or FA extraction for 7 days in succession (once per day): the control group treated with saline, low-dose group received 10 g kg-1day-1 FA extraction, medium-dose group received 20 g kg-1day-1 FA extraction and high-dose group received 30 g kg-1day-1 FA extraction. Rats were anesthetized with chloral hydrate (3–4 ml kg-1 bw) and then sacrificed 1 h later after the last administration. The blood samples were collected into non-heparinized tubes by cardiac puncture. Followed centrifugation at 2500 rpm min-1 for 20 min at 4°C, which the serums were separated, the blood samples were kept at room temperature for about 2 h. The serums were decanted and then filled with 0.22 μm cellulose acetate membrane twice. After that, the serums were calefied by 56°C water for 30 min and then stored at -20°C until use. Liver sample from each animal was excised, perfused with ice-cold 0.9% (w/v) sodium chloride to remove blood, weighted, and stored at -80°C.
Western Blot Analysis
The liver samples were lysed with RIPA/PMSF (100:1, v/v) lysis buffer for 30 min on ice, following being grounded into powder with the help of liquid nitrogen. Total protein was separated by centrifugation at 12000 rpm min-1 at 4°C for 30 min. The protein concentrations were measured by using the BCA protein assay kit according to the manufacturer’s protocol. Protein fully mixed with loading buffer (4:1, v/v) and then denatured at 100°C for 5 min. Equal amounts of protein (64 μg lane-1) were separated by SDS–PAGE gel with 5% (v/v) stacking gel and 10% (v/v) resolving gel. Proteins were transferred onto PVDF membranes and blocked with 5% BSA in Tris-buffered saline with 0.1% Tween-20 (TBST) for 2 h at room temperature. Subsequently, membranes were incubated overnight at 4°C with 1:1000, 1:200, 1:4000, and 1:5000 dilutions of monoclonal antibodies for CYP2E1, CYP1A2, CYP3A4, and β-actin, respectively. Membranes were washed thrice with TBST and then incubated for 1 h at room temperature with 1:5000 dilutions of anti-rabbit horseradish peroxidase-conjugated secondary antibodies for CYP2E1, CYP1A2, and CYP3A4 and with a 1:5000 dilution of anti-mouse horseradish peroxidase-conjugated secondary antibody for β-actin. Again, membranes were rinsed three times. Finally, proteins were detected with an enhanced chemiluminescence (ECL) kit, followed imaging through ChemiDocTM XRS Imaging System (Bio-Rad Laboratories, United States). The protein bands were scanned by Image LabTM software (version 4.0, Bio-Rad Laboratories, United States). Quantity One® software was used for the densitometric analysis of the bands. All isoforms were normalized to β-actin and data were presented as the relative protein expression.
Real-Time PCR Analysis
Total RNAs were extracted from the rat liver samples with Trizol Reagent in accordance with the manufacturer’s instruction. The concentration of RNA was quantified and the quality of RNA was assessed through the ratio of the absorbance at 260 and 280 nm. Subsequently, 2 μg of RNA was reversely transcripted to cDNA using RevertAid First Strand cDNA Synthesis kit, and then the cDNA was amplified using All-in-OneTM qPCR Mix Kit. The sequences of the primers are shown in Table 1. The reactions were conducted with a total reaction volume of 20 μl containing 2 μl of template diluted (1:10). The program of RT-PCR was set as follow: 95°C for 10 min, 40 cycles of 95°C for 15 s, 57°C for 20 s, and 72°C for 45 s. The relative mRNA expression was analyzed by the 2-ΔΔCt method.
Table 1.
The RT-PCR oligonucleotide primers in rat.
| Gene | Primer | Sequence (5′–3′) | PCR product (bp) |
|---|---|---|---|
| CYP2E1 | Forward | CCTACATGGATGCTGTGGTG | 171 |
| Reverse | CTGGAAACTCATGGCTGTCA | ||
| CYP3A4 | Forward | TCTGTGCAGAAGCATCGAGTG | 253 |
| Reverse | TGGGAGGTGCCTTATTGGG | ||
| CYP1A2 | Forward | TGTCACCTCAGGGAATGCT | 212 |
| Reverse | GACCACCGTTGTCTTTGTAG | ||
| β-actin | Forward | TCGTGCGTGACATTAAAGAG | 134 |
| Reverse | ATTGCCGATAGTGATGACCT |
Effect of FA on the CYPs Activities in Rats
The rats were randomly divided into two subgroups (six each) and then orally administrated saline or FA extraction for 7 days in succession (once per day): the control group treated with saline and experimental group received 30 g kg-1day-1 FA extraction. On the 8th day morning, 5 ml kg-1 of probe cocktail solution, containing caffeine (10 mg kg-1), dapsone (10 mg kg-1), and chlorzoxazone (20 mg kg-1) suspended in CMC-Na solution, was then given to the rats in each group by intragastric administration. Blood samples were collected into heparinized tubes from the rat tail vein at a range of time-points (0, 5, 15, 30, 45, 60, 90, 120, 180, 240, 360, 480, 720, and 1440 min) after oral administration of three probe drugs, followed immediate centrifugation at 3500 rpm min-1 for 15 min to obtain 100 μl rat plasma samples. These were stored at -20°C until analysis.
To each 100 μl of rat plasma sample, the internal standard (30 μl 7.6 μg ml-1 sulfamethoxazole) was added before extraction with 370 μl acetonitrile, vortexing for 1 min and centrifugation at 5000 rpm min-1 for 5 min. A total of 400 μl supernatant was evaporated to dryness under a stream of nitrogen. The residue was dissolved in 50% methanol followed centrifugation at 12000 rpm min-1 for 5 min. A total of 10 μl of the solution was injected into UPLC analysis.
The standards of these components (caffeine, dapsone, and chlorzoxazone) were obtained from the National Institute for Food and Drug Control. A mixed stock standard solutions were added to blank plasma to yield seven different concentrations: for caffeine, they were 16.667, 8.333, 4.167, 2.083, 1.042, 0.521, and 0.260 μg ml-1; for dapsone, they were 3.300, 1.650, 0.825, 0.413, 0.206, 0.103, and 0.052 μg ml-1; for chlorzoxazone, they were 22.000, 11.000, 5.500, 2.750, 1.375, 0.688, and 0.344 μg ml-1, and for sulfamethoxazole, they were 119.000, 59.500, 29.750, 14.875, 7.438, 3.719, and 1.859 μg ml-1. The samples were extracted and analyzed according to the sample preparation procedures described above.
Chromatographic analysis was carried out on a Waters Acquity UPLC system, which equipped with a quaternary solvent delivery system, an auto-sampler fitted with a 10 μl loop and a PDA optical detector for ultraviolet wavelengths (190–480 nm). The separation was performed on Waters BEH (R) C18 (50 mm × 2.1 mm, 1.7 μm). The mobile phase consisted of acetonitrile (A) and 0.5% acetic acid in water (B). The elution gradient was as follows: 0–2 min, 20–30%A; 2–3 min, 30–40%A; 3–4 min, 40–40%A; 4–5 min, 40–50%A; 5–7 min, and 50–20%A. The flow rate was 0.2 ml min-1. The column temperature was maintained at 40°C. UV absorbance wavelengths were detected at 237, 293, and 281 nm for caffeine, dapsone, and chlorzoxazone, respectively.
Effect of FA-Medicated Serum on CYPs mRNA Expression in HepG2 Cells
Serum Sample Preparation for UPLC Analysis
Naringenin (catalog No. 130330), hesperetin (catalog No. 130816), and merazin hydrate (catalog No. 20090118) in the FA-medicated serum were monitored by UPLC-PDA method. The standards of these components that were purchased from Chengdu must be Bio-Technology Co., Ltd. Stock solutions were separately prepared by dissolving the accurately weighed these standard reference compounds with methanol. A mixed stock solution was obtained by mixing the three stock solution above, and giving a final concentration of 3.555 μg ml-1 for naringenin, 0.236 μg ml-1 for hesperetin, and 3.300 μg ml-1 for meranzin hydrate, respectively. All the solutions were stored at 4°C refrigerator until use.
An aliquot (200 μl) of low-dose group rat serum was precipitated protein by vortexing vigorously with acetonitrile (500 μl) for 2 min. The supernatant was decanted and then evaporated to dryness under a stream of nitrogen after centrifugation at 10000 g for 15 min at 4°C. The residue was dissolved in methanol (100 μl), vortexing for 3 min, and then filtered through a 0.2-μm film. Following being ultrasonated for 1 min and repeating the same centrifugation procedure, an aliquot (10 μl) was injected onto the UPLC column for analysis.
Chromatographic analysis was conducted on the Waters Acquity UPLC system. The mobile phase consisted of acetonitrile (A) and 0.5% acetic acid in water (B). The gradient program was as follows: 0–2 min, 20–20% A; 2–4 min, 20–30% A; 4–5 min, 30–30% A; 5–7 min, 30–40%; 7–8 min, 40–40% A; 8–10 min, 40–20% A. The flow rate was 0.2 ml min-1, and the column temperature was set at 40°C. UV absorbance was detected at 284 nm using a photodiode array detector.
Cell Culture
HepG2 cells were cultured in the humidified incubator with a 5% CO2 atmosphere at 37°C. The medium used for cell culture, which was changed regularly, consisted of 89% DMEM, 0.5% penicillin (100 units ml-1), 0.5% streptomycin (100 g ml-1), and 10% fetal bovine serum. Cells were used for experiments when the confluence reached to 90%.
MTT Assay
The cytotoxic effects of FA in HepG2 cells were assessed by the MTT assay. Briefly, HepG2 cells were seeded at a density of 10,000 cells per well in 96-well cell culture plates. After 24 h treatment, the cell culture mediums were discarded and replaced with the new medium containing 10% rat serum (saline-treated rat serum, FA-treated rat serums) instead of 10% FBS. Culture medium without HepG2 cells was considered as the blank group and culture medium containing saline-treated rat serum with HepG2 cells as control group. After 24 h treatment in the cell incubator, MTT (5 mg ml-1, diluted with PBS buffer) were added into each well. Following another 4 h of incubation, the supernatants were removed and DMSO was added. After the plate was shaken for 10 min, the absorbance was recorded at the wavelength of 570 nm by an enzyme-labeling instrument:
Real-Time PCR Analysis
HepG2 cells were plated in 6-well plates and then divided into four groups as 10% saline-treated rat serum group (control group), 10% low dosage FA-medicated serum group, 10% medium dosage FA-medicated serum group, and 10% high dosage FA-medicated serum group. Cells were pre-incubated with the corresponding sera for 24 h. At the terminal of treatment, total RNAs were extracted with Trizol Reagent in accordance with the manufacturer’s instruction. The concentration of RNA was quantified and the quality of RNA was assessed through the ratio of the absorbance at 260 and 280 nm. Subsequently, 2 μg of RNA was reversely transcripted to cDNA using RevertAid First Strand cDNA Synthesis kit, and then the cDNA was amplified using All-in-OneTM qPCR Mix Kit. The sequences of the primers are shown in Table 2. The reactions were conducted with a total reaction volume of 20 μl containing 2 μl of template diluted (1:10). The program of RT-PCR was set as follow: 95°C for 10 min, 40 cycles of 95°C for 15 s, 58°C for 20 s, and 72°C for 45 s. The relative mRNA expression was analyzed by the 2-ΔΔCt method.
Table 2.
The RT-PCR oligonucleotide primers in HepG2 cells.
| Gene | Primer | Sequence (5′–3′) | PCR product (bp) |
|---|---|---|---|
| CYP2E1 | Forward | CTCGTGGAAATGGAGAAGGA | 112 |
| Reverse | TTGTGCTGGTGGTCTCTGTC | ||
| CYP3A4 | Forward | CAAGACCCCTTTGTGGAAAA | 184 |
| Reverse | CGAGGCGACTTTCTTTCATC | ||
| CYP1A2 | Forward | GGGCACTTCGACCCTTACAA | 63 |
| Reverse | GCACATGGCACCAATGACG | ||
| β-actin | Forward | GGGCACGAAGGCTCATCATT | 295 |
| Reverse | AGTCGGTTGGAGCGAGCATC |
Statistical Analysis
Statistical analysis was performed with the SPSS 17.0 (SPSS Inc., Chicago, IL, United States). Data are expressed as mean ± SD. Pharmacokinetic analysis of data was calculated by DAS 2.0 (Mathematical Pharmacology Professional Committee of China, Shanghai, China) and statistically significant difference between groups was assessed by a two-tailed, two sample t-test. Other data were analyzed by one-way analysis of variance (ANOVA). A P-value less than 0.05 were considered as the statistical significant difference.
Results
Effect of FA on CYPs Protein Expressions in Rat Liver
As demonstrated in Figures 1, 2, Western blot results of CYP1A2, CYP2E1, and CYP3A4 indicated visible upregulation of protein expression in rat liver compared to saline-treated group. CYP1A2 and CYP3A4 protein expression was significantly induced in all treatment conditions. Similarly, the protein expression of CYP2E1 was significantly up-regulated.
FIGURE 1.
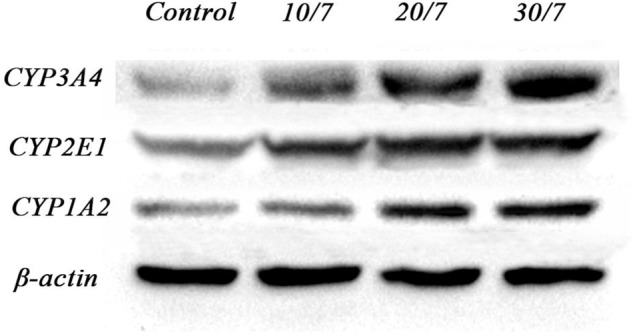
Grayscale plot of protein expression ration of CYP1A2, CYP3A4, and CYP2E1 in rat liver determined by Western Blot. Control, the control group treated with saline; 10/7, low-dose group received 10 g kg-1day-1 FA extraction; 20/7, medium-dose group received 20 g kg-1day-1 FA extraction; and 30/7, high-dose group received 30 g kg-1day-1 FA extraction.
FIGURE 2.
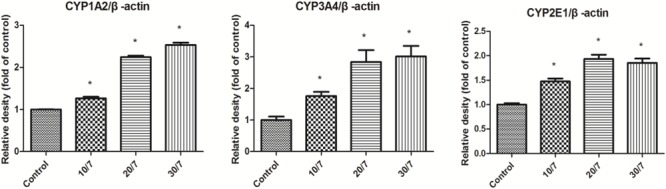
Relative protein expression ration of CYP1A2, CYP3A4, and CYP2E1 in rat liver determined by Western Blot. Control, the control group treated with saline; 10/7, low-dose group received 10 g kg-1day-1 FA extraction; 20/7, medium-dose group received 20 g kg-1day-1 FA extraction; and 30/7, high-dose group received 30 g kg-1day-1 FA extraction. Values are means ± SD and ∗P < 0.05 compared with control group.
Effect of FA on CYPs mRNA Expression in Rat Liver
The effects of FA on CYP1A2, CYP2E1, and CYP3A4 mRNA expression in rat liver were determined by RT-PCR method. As shown in Figure 3, compared with the control group, the mRNA expression of CYP1A2 was significantly induced, especially at the high dosage group; FA seemed to increase CYP3A4 mRNA expression in a dose-dependent manner and statistically significant increase were observed at all treatment conditions, which is consistent with the corresponding CYP protein expression. However, there were not significant changes in CYP2E1 mRNA expression.
FIGURE 3.
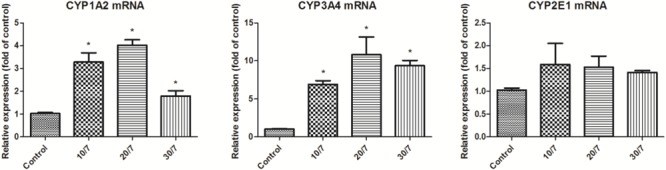
CYP1A2, CYP3A4, and CYP2E1 mRNA expression in rat liver after orally administration of FA. Control, the control group treated with saline; 10/7, low-dose group received 10 g kg-1day-1 FA extraction; 20/7, medium-dose group received 20 g kg-1day-1 FA extraction; and 30/7, high-dose group received 30 g kg-1day-1 FA extraction. Values are means ± SD and ∗P < 0.05 compared with control group.
Effect of FA on the CYPs Activities in Rats
The concentrations of caffeine, dapsone, and chlorzoxazone in rat plasma were determined by a sensitive and simple UPLC method. CYP1A2, CYP3A4, and CYP2E1 activities were evaluated by comparing pharmacokinetic parameters of caffeine, dapsone, and chlorzoxazone in control and experimental group. The main pharmacokinetic parameters of caffeine, dapsone, and chlorzoxazone in rats are presented in Table 3. Mean plasma concentration–time curves of caffeine, dapsone, and chlorzoxazone are presented in Figure 4. As demonstrated in Table 3, compared with the control group, the t1/2, Cmax, AUC(0-∞), and MRT(0-∞) of caffeine and dapsone in experimental group were decreased significantly (P < 0.05); CL of caffeine and dapsone in experimental group were increased significantly (P < 0.05). The results indicated that the metabolisms of caffeine and dapsone in experimental group were evidently accelerated and that potential induction of rat hepatic CYP1A2 and CYP3A4 activities was evidence-based in presence of FA. However, no significant differences for the pharmacokinetic parameters of chlorzoxazone (P > 0.05) between the control group and the experimental group were observed, which indicated that FA was not able to induce or inhibit rat hepatic CYP2E1 activity in vivo.
Table 3.
Main pharmacokinetic parameters of caffeine, dapsone, and chlorzoxazone in rat plasma.
| Probe drug | Pharmacokinetics | Control group | Experimental group |
|---|---|---|---|
| Caffeine | t1/2(h) | 6.76 ± 3.59 | 4.06 ± 1.99∗ |
| Cmax (mg L-1) | 9.92 ± 1.82 | 7.06 ± 1.66∗∗ | |
| AUC(0-∞) (mg L-1 h) | 77.50 ± 16.88 | 31.75 ± 5.35∗∗ | |
| MRT(0-∞) (h) | 8.05 ± 2.02 | 5.50 ± 1.59∗ | |
| CL (L h-1 kg-1) | 0.13 ± 0.03 | 0.32 ± 0.06∗∗ | |
| Dapsone | t1/2 (h) | 11.58 ± 4.72 | 8.59 ± 3.52∗ |
| Cmax (mg L-1) | 1.74 ± 0.39 | 2.01 ± 0.69 | |
| AUC(0-∞) (mg L-1 h) | 33.52 ± 8.10 | 22.83 ± 3.46∗∗ | |
| MRT(0-∞) (h) | 17.94 ± 6.60 | 12.1 ± 4.03 | |
| CL (L h-1 kg-1) | 0.31 ± 0.08 | 0.45 ± 0.06∗∗ | |
| Chlorzoxazone | t1/2 (h) | 1.29 ± 0.95 | 0.98 ± 0.63 |
| Cmax (mg L-1) | 10.90 ± 2.25 | 9.56 ± 1.50 | |
| AUC(0-∞) (mg L-1 h) | 39.74 ± 3.97 | 22.71 ± 3.91∗ | |
| MRT(0-∞) (h) | 3.42 ± 0.25 | 4.53 ± 1.58 | |
| CL (L h-1 kg-1) | 0.51 ± 0.05 | 0.90 ± 0.16∗ |
t1/2, half-life; Cmax, the peak or maximum concentration; AUC(0-∞), area under a curve extrapolated to infinity; MRT(0-∞), mean residence time; CL, the total body clearance. Values are presented as mean ± SD (n = 6).∗P < 0.05 when compared with related parameters of control group. ∗∗P < 0.01 when compared with related parameters of control group.
FIGURE 4.
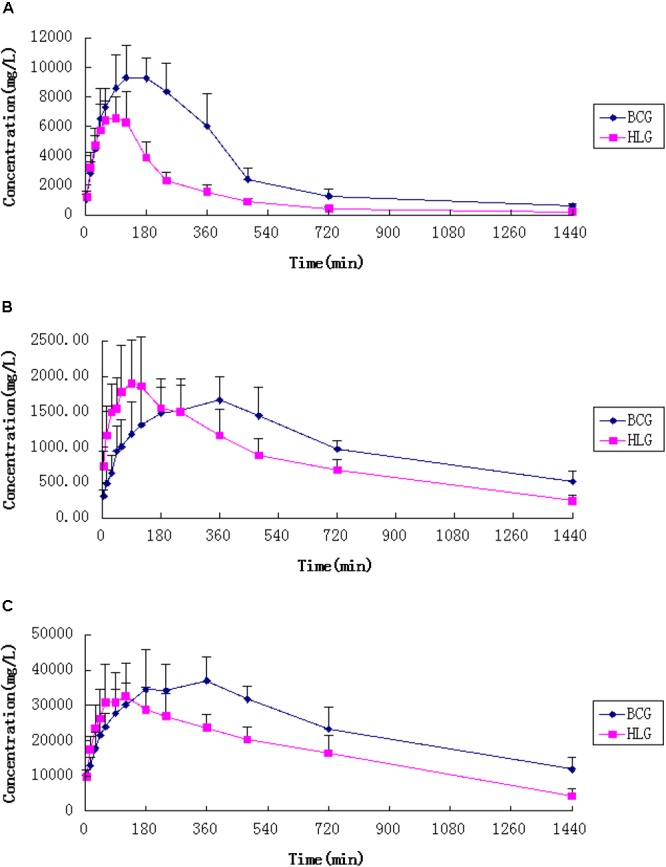
Mean plasma concentration-time curves of caffeine (A), dapsone (B), and chlorzoxazonee (C) in untreated and FA extract pre-treated rats (n = 6). Blank control group (BCG) received saline for 7 days; HLG (experimental group) received 30 g kg-1day-1 FA extraction. Error bars represent SD.
UPLC Analysis of FA-Medicated Serum
The UPLC chromatogram implied that there were complex components with several peaks at different retention times in the FA-medicated serum (Figure 5), those components including prototype drug component and their metabolites. The retention time of reference standards (meranzin hydrate, naringenin, and hesperetin) was 5.3, 7.8, 8.1 min, respectively. With regard to retention time and UV wavelength of reference substances, it was verified that the FA-medicated serum contained meranzin hydrate, naringenin, and hesperetin.
FIGURE 5.
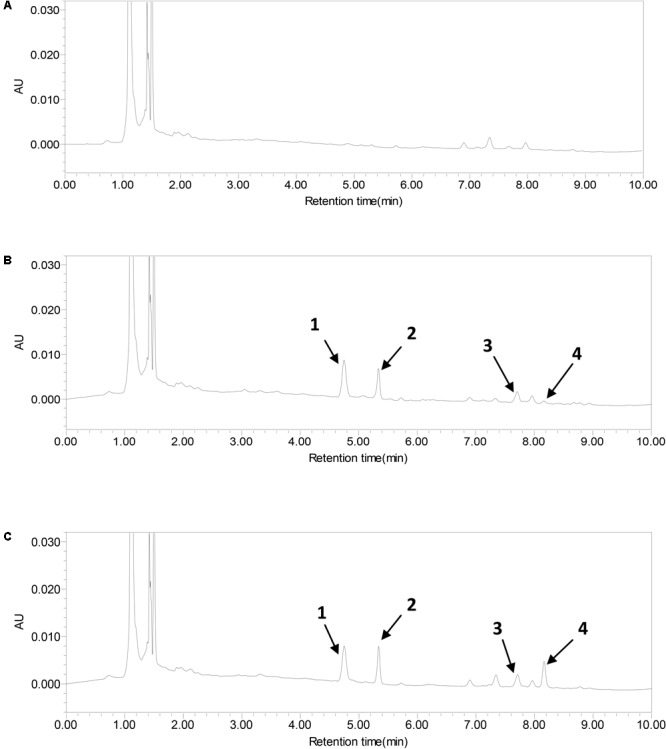
UPLC-PAD chromatograms of sulfamethoxazole (1), meranzin hydrate (2), naringenin (3), and hesperetin (4) in serum. (A) Blank serum, (B) FA-mediated serum, and (C) blank serum added the standard substances.
Effects of FA-Medicated Serum on Cell Viability
The MTT assay was applied to assess the effect of FA-medicated serum on HepG2 cell viability, which was presented in Table 4. Results showed that low dosage, medium dosage, and high dosage FA-medicated serum had no statistical significant influence on cell viability (P > 0.05) compared to the control group. Thus, the serum could be used for further study.
Table 4.
Effects of FA-medicated serums on cell viability of HepG2 cells.
| Concentration (g kg-1) | FA-medicated serum |
Control group | ||
|---|---|---|---|---|
| l0 (low dosage) | 20 (medium dosage) | 30 (high dosage) | / | |
| OD value Cell viability (%) | 0.897 ± 0.166 | 0.838 ± 0.113 | 0.944 ± 0.144 | 0.997 ± 0.053 |
| 89.98 | 84.07 | 94.66 | 100.00 | |
| P-value | 0.488245 | 0.144198 | 0.654195 | / |
The absorbance value at 570 nm (mean ± SD, n = 5).
Expression of CYP450 Isoenzymes mRNA Expression in HepG2 Cells Cultured With FA-Mediated Serum
The RT-PCR analysis, which was shown in Figure 6, was performed to determine the effects of FA-medicated serums on CYP1A2, CYP3A4, and CYP2E1 mRNA expression in HepG2 cells. FA-medicated serum seemed to increase CYP1A2 mRNA expression in a dose-dependent manner; statistically significant increase was observed at the high dosage group. The mRNA expression of CYP3A4 was significantly induced when HepG2 cells were treated with 10% low and medium dosage FA-medicated serum, except 10% high dosage FA-medicated serum. However, there was very small, not significant change in CYP2E1 mRNA expression.
FIGURE 6.
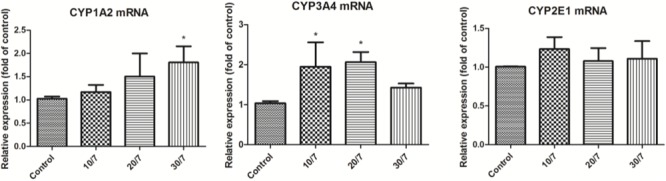
CYP1A2, CYP3A4, and CYP2E1 mRNA expression in HepG2 cells cultured with FA-mediated serum using real-time PCR. Control, 10% saline-treated rat serum group; 10/7, 10% low dosage FA-medicated serum group; 20/7, 10% medium dosage FA-medicated serum group; and 30/7, 10% high dosage FA-medicated serum group. Results are expressed as mean ± SD and ∗P < 0.05 compared with the control.
Discussion
It is estimated that up to 80% of the population in developing countries use traditional herbal medicine for primary health care (Picking et al., 2011). In other word, Herbal medicines play important roles in the primary health care of individuals and communities. But there is a potential risk of unexpected drug–drug interactions when patients administrate herbs and prescribed modern medication simultaneously without informing their physician of their herb use (Ooi et al., 2011). The classical herb implicated with drug–drug interactions is St. John’s wort (Sim and Levine, 2010; Ooi et al., 2011). Pharmacokinetic interaction is one category of drug–drug interaction (Pal and Mitra, 2006), which mainly attributed to the induction or inhibition of specific CYPs. The CYPs superfamily is one of the most important drug-metabolizing enzyme systems and involves in the biotransformation of a large number of exogenous and endogenous compounds (Wang et al., 2015). Depending on how the drugs interact with CYPs, it can be beneficial by enhancing blood levels or detrimental leading to therapeutic failure (Pal and Mitra, 2006). For example, chronic administration of certain herbal products can enhance the production of CYPs, resulting in lowering plasma concentrations due to either higher metabolism and/or higher efflux. Such interactions may lead to lower bioavailability and subtherapeutic plasma drug concentrations (Kumar et al., 2010). In this study, we investigated the effects of the herb FA on hepatic CYP1A2, CYP2E1, and CYP3A4.
In our study, FA significantly induced CYP2E1 protein expression, but did not appear to have significant effect on the corresponding mRNA expression in rat liver. Previous studies have proved that the expression of specific mRNAs and subsequent accumulation of corresponding proteins are not always in accordance (Nakaminami et al., 2014). The research has demonstrated that the CYP3A4 mRNA level correlated significantly to the CYP3A4 protein level, which was not the case for CYP2E1 (Sumida et al., 1999). It has also been demonstrated that CYPs in the liver usually are regulated at the transcriptional level except for CYP2E1. Despite that the mRNAs are the source of protein content, protein expression, namely CYP2E1, can be regulated at various levels including pre-transcription, transcription, pre-translation, translation, and post-translation (Sumida et al., 1999). This may explain our results that CYP2E1 mRNA expression and its corresponding protein expression is not completely consistent. It is reported that the most common mechanism of CYP450 induction is transcriptional activation resulting in the increased protein synthesis of CYP enzymes (Døssing et al., 1983; Han et al., 2014). Thus, For CYP2E1, our result indicates that FA is unlikely to significantly affect CYP2E1 until the clinical researches are conducted. For CYP1A2 and CYP 3A4, the present results showed that FA up-regulated CYP1A2 and CYP3A4 significantly not only from the protein expression but also from the mRNA expression in rat liver. This suggests that FA is potential to induce CYP1A2 and CYP3A4 before completing the clinic trial. Another reason for our conclusions was based on the fact that FA had the potential to induce rat hepatic CYP1A2 and CYP3A4 activities responsible for relevant alterations of probe drug plasma levels, and was not able to induce or inhibit rat hepatic CYP2E1 activity in the experiment. These imply that the potential risk of drug–drug interaction will occur when FA is consumed accompanied with CYP1A2 or CYP3A4 substrates. Conversely, there is low probability of pharmacokinetic interaction between FA and substrates of CYP2E1.
The CYPs mRNA expression in HepG2 cell is based on HepG2 cell model, serum pharmacological method, UPLC method, and MTT assay for reasons as follow: first, HepG2 cells, the human liver cancer cell with different gene expression profiles, are known to constitutively express CYP1A2, CYP2E1, and CYP3A4 (Ooi et al., 2011). Besides, HepG2 cell model is easy to obtain, control, and perform without the inter-individual and inter-species variation and interpretation difficulties, which could accurately evaluate the potential inductive effect for drugs (Cui et al., 2014). Second, Serum pharmacological method is frequently adapted to the study of herb medicines, as it not only eliminates the interferences of the physical and chemical characters of crude drugs on the experimental results, but also provides the experimental conditions similar to the in vivo environment (Yin et al., 2014). To replicate the required experimental environment, the 10% (v/v) FA-medicated serum, which is proved statistical non-toxic by MTT assay (Table 4) was used for our study. Third, UPLC-PDA-Q-TOF/MS analysis of constituents absorbed into blood of FA extract indicates that flavonoid glycosides, including naringin, hesperidin, and neohesperidin were main components absorbed in vivo (Ma et al., 2011). It has been reported that the AUC0-t of naringenin and hesperetin were much higher than those of flavanone glycosides in rat plasma following oral administration of FA extract, which might result from the hydrolysis of flavanone glycosides (naringin, hesperidin, and neohesperidin) (Tong et al., 2012). Meranzin hydrate was considered as one major component of FA and induced similar effect to FA on intestinal motility (Huang et al., 2011). Hence, in our present study, naringenin, hesperetin, and meranzin hydrate were selected to be detected as the bioactive compounds in FA-mediated rat serum (Figure 1). Fourth, the result of MTT assay showed that FA-medicated serum at the three different concentrations did not exhibit significant cytotoxicity against HepG2 cells, which exclude the effect of the variable-the activity and number of HepG2 cells on the result of PCR analysis. In our study, FA-medicated serum increased CYP1A2 and CYP3A4 mRNA expression with statistically significant difference compared to the control group, while there was not significant change in CYP2E1 mRNA expression. This result supported the conclusion get in rat above.
A previous study showed that water extract of FA immature increased CYP3A4 protein expression and ethanol extract of FA immature induced CYP3A4 expression via induction of PXR expression (Okada et al., 2017). They speculated that narirutin might be the key constituent increasing PXR expression and CYP3A4 protein expression. It is well known that the efficacy of herbs is significantly correlated with active constituents in herbs. Narirutin is also one of the major flavonoids in FA and thought to be biological active (Huang et al., 2012). It has similar effects to FA to some extent (Huang et al., 2012). Thus, narirutin might be a main compound contributing to the slight induction of FA on CYPs. This needs further experiments to test the hypothesis.
The CYP enzyme induction, one of the common cases of metabolic enzymes related to drug–drug interaction beside the drug metabolism inhibition, causes serious clinical consequences (Cui et al., 2014). For drugs that produce therapeutic effects primarily by the prototype drug, induction may result in a reduced therapeutic efficacy of co-administrated drugs, or even lead to therapy failure (Lin, 2006). For that, the dosage of drugs should be modified within their safe range clinically. For example, rifampin interacts with warfarin resulted from its enzyme inducer activity on CYP2C9, CYP3A4, CYP1A2, and CYP2C19. There was a case report that a female on anticoagulation increased warfarin dose from 52.5 to 210 mg/week with a target INR during the 6-week treatment with rifampin for her infective endocarditis and she was stable on the warfarin dose of 80 mg/week 5 weeks after the end of rifampin therapy (Fahmi et al., 2016). Multiple dose adjustment may occur when FA is combined with CYP1A2 or CYP3A4 substrates with a narrow therapeutic index. Besides, induction may increase the risk of metabolite-induced toxicity by increasing reactive metabolite formation, such as CYP1A induction (Lin, 2006).
Conclusion
Our study shows that FA may be a potential inducer of CYP1A2, CYP3A4, and may not be able to affect CYP2E1 until the clinic researches are performed. Thus, caution should paid to reduce adverse drug–drug interaction when FA is administrated combined with CYP1A2 or CYP3A4 substrates.
Data Availability Statements
All the relevant data are contained within the manuscript.
Author Contributions
XQ contributed the conception and the design of this study. LZ and MC conducted the experiments. LZ and XQ wrote the main manuscript text. All the authors participated in performing the laboratory analyses and interpreting the data. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was supported by the China Postdoctoral Science Foundation (Grant No. 2015M572254), National Natural Science Foundation of China (Grant Nos. 81202807 and 81703963), and Prominent Traditional Chinese Medicine Doctor Suiyu Hu’s Inheritance Studio.
References
- Bièche I., Narjoz C., Asselah T., Vacher S., Marcellin P., Lidereau R., et al. (2007). Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet. Genomics 17 731–742. 10.1097/FPC.0b013e32810f2e58 [DOI] [PubMed] [Google Scholar]
- Cui H. M., Zhang Q. Y., Wang J. L., Chen J. L., Zhang Y. L., Tong X. L. (2014). In vitro studies of berberine metabolism and its effect of enzyme induction on HepG2 cells. J. Ethnopharmacol. 158 388–396. 10.1016/j.jep.2014.10.018 [DOI] [PubMed] [Google Scholar]
- Døssing M., Pilsgaard H., Rasmussen B., Poulsen H. E. (1983). Time course of phenobarbital and cimetidine mediated changes in hepatic drug metabolism. Eur. J. Clin. Pharmacol. 25 215–222. 10.1007/BF00543794 [DOI] [PubMed] [Google Scholar]
- Fahmi A. M., Abdelsamad O., Elewa H. (2016). Rifampin-warfarin interaction in a mitral valve replacement patient receiving rifampin for infective endocarditis: a case report. Springerplus 5:8. 10.1186/s40064-015-1653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R., Huang X., Wang Y., Chen X., Ren P., Ji H., et al. (2012). Ethnopharmacokinetic- and activity-guided isolation of a new antidepressive compound from Fructus aurantii found in the traditional Chinese medicine Chaihu-Shugan-San: a new approach and its application. Evid. Based Complement. Alternat. Med. 2012:607584. 10.1155/2012/607584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. Q., Taniguchi M., Chen Q. Y., Baba K., Yamazoe Y. (2001). Inhibitory potential of herbal medicines on human cytochrome P450-mediated oxidation: properties of umbelliferous or citrus crude drugs and their relative prescriptions. Jpn. J. Pharmacol. 85 399–408. 10.1254/jjp.85.399 [DOI] [PubMed] [Google Scholar]
- Han S. Y., Zhao H. Y., Zhou N., Zhou F., Li P. P. (2014). Marsdenia tenacissima extract inhibits gefitinib metabolism in vitro by interfering with human hepatic CYP3A4 and CYP2D6 enzymes. J. Ethnopharmacol. 151 210–217. 10.1016/j.jep.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Hodek P., Trefil P., Stiborová M. (2002). Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Interact. 139 1–21. 10.1016/S0009-2797(01)00285-X [DOI] [PubMed] [Google Scholar]
- Huang W., Huang X., Xing Z., Qiu X., Wang Y., Fan R., et al. (2011). Meranzin hydrate induces similar effect to Fructus aurantii on intestinal motility through activation of H1 histamine receptors. J. Gastrointest. Surg. 15 87–96. 10.1007/s11605-010-1374-9 [DOI] [PubMed] [Google Scholar]
- Huang W., Xiong Z. H., Huang X., Chen X., Liu W. P., Wang Y., et al. (2012). Simultaneous UPLC analysis of three major flavonoids in granule decoctions of Fructus aurantii-type formulae. Pharmazie 67 586–589. [PubMed] [Google Scholar]
- Huang Y. T., Wang G. F., Chen C. F., Chen C. C., Hong C. Y., Yang C. (1995). Fructus aurantii reduced portal pressure in portal hypertensive rats. Life Sci. 57 2011–2020. 10.1016/0024-3205(95)02195-O [DOI] [PubMed] [Google Scholar]
- Kang M., Kim J. H., Cho C., Chung H. S., Kang C. W., Kim Y., et al. (2007). Anti-ischemic effect of Aurantii Fructus on contractile dysfunction of ischemic and reperfused rat heart. J. Ethnopharmacol. 111 584–591. 10.1016/j.jep.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Chung H. S., Kang M., Kim Y., Kim Y. S., Bae H. (2011). Anti-diabetic effect of standardized herbal formula PM021 consisting of mori folium and Aurantii fructus on type II diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Diabetes Res. Clin. Pract. 93 198–204. 10.1016/j.diabres.2011.03.037 [DOI] [PubMed] [Google Scholar]
- Kumar Y. S., Adukondalu D., Sathish D., Vishnu Y. V., Ramesh G., Latha A. B., et al. (2010). P-Glycoprotein- and cytochrome P-450-mediated herbal drug interactions. Drug Metabol. Drug Interact. 25 3–16. 10.1515/DMDI.2010.006 [DOI] [PubMed] [Google Scholar]
- Li X., Xiao H., Liang X., Shi D., Liu J. (2004). LC–MS/MS determination of naringin, hesperidin and neohesperidin in rat serum after orally administrating the decoction of Bupleurum falcatum L. and Fractus aurantii. J. Pharm. Biomed. Anal. 34 159–166. 10.1016/j.japna.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Lin J. H. (2006). CYP induction-mediated drug interactions: in vitro assessment and clinical implications. Pharm. Res. 23 1089–1116. 10.1007/s11095-006-0277-7 [DOI] [PubMed] [Google Scholar]
- Liu L., Cheng Y., Zhang H. (2004). Phytochemical analysis of anti-atherogenic constituents of Xue-Fu-Zhu-Yu-Tang using HPLC-DAD-ESI-MS. Chem. Pharm. Bull. 52 1295–1301. 10.1248/cpb.52.1295 [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhang J., Gao W., Liu C. (2013). Antinociceptive activity and chemical composition of Wei-Chang-An-Wan extracts. Pharm. Biol. 51 790–797. 10.3109/13880209.2013.766893 [DOI] [PubMed] [Google Scholar]
- Ma C., Gao W., Gao Y., Man S., Huang L., Liu C. (2011). Identification of chemical constituents in extracts and rat plasma from Fructus aurantii by UPLC-PDA-Q-TOF/MS. Phytochem. Anal. 22 112–118. 10.1002/pca.1252 [DOI] [PubMed] [Google Scholar]
- Nakaminami K., Matsui A., Nakagami H., Minami A., Nomura Y., Tanaka M., et al. (2014). Analysis of differential expression patterns of mRNA and protein during cold-acclimation and de-acclimation in Arabidopsis. Mol. Cell. Proteomics 13 3602–3611. 10.1074/mcp.M114.039081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Murakami A., Urushizaki S., Matsuda M., Kawazoe K., Ishizawa K. (2017). Extracts of immature orange (Aurantii fructus immaturus) and Citrus Unshiu Peel (Citri unshiu pericarpium) induce P-glycoprotein and cytochrome P450 3A4 expression via upregulation of pregnane X receptor. Front. Pharmacol. 8:84. 10.3389/fphar.2017.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi J. P., Kuroyanagi M., Sulaiman S. F., Muhammad T. S., Tan M. L. (2011). Andrographolide and 14-deoxy-11, 12-didehydroandrographolide inhibit cytochrome P450s in HepG2 hepatoma cells. Life Sci. 88 447–454. 10.1016/j.lfs.2010.12.019 [DOI] [PubMed] [Google Scholar]
- Pal D., Mitra A. K. (2006). MDR- and CYP3A4-mediated drug-drug interactions. J. Neuroimmune Pharmacol. 1 323–339. 10.1007/s11481-006-9034-2 [DOI] [PubMed] [Google Scholar]
- Picking D., Younger N., Mitchell S., Delgoda R. (2011). The prevalence of herbal medicine home use and concomitant use with pharmaceutical medicines in Jamaica. J. Ethnopharmacol. 137 305–311. 10.1016/j.jep.2011.05.025 [DOI] [PubMed] [Google Scholar]
- Sim S. N., Levine M. A. (2010). An evaluation of pharmacist and health food store retailer’s knowledge regarding potential drug interactions associated with St. John’s wort. Can. J. Clin. Pharmacol. 17 e57–e63. [PubMed] [Google Scholar]
- Sumida A., Kinoshita K., Fukuda T., Matsuda H., Yamamoto I., Inaba T., et al. (1999). Relationship between mRNA levels quantified by reverse transcription-competitive PCR and metabolic activity of CYP3A4 and CYP2E1 in human liver. Biochem. Biophys. Res. Commun. 262 499–503. 10.1006/bbrc.1999.1233 [DOI] [PubMed] [Google Scholar]
- Tong L., Zhou D., Gao J., Zhu Y., Sun H., Bi K. (2012). Simultaneous determination of naringin, hesperidin, neohesperidin, naringenin and hesperetin of Fractus aurantii extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 58 58–64. 10.1016/j.jpba.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Tydén E., Tjälve H., Larsson P. (2014). Gene and protein expression and cellular localisation of cytochrome P450 enzymes of the 1A, 2A, 2C, 2D and 2E subfamilies in equine intestine and liver. Acta Vet. Scand. 56:69. 10.1186/s13028-014-0069-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu S., Chen Z., Zhang H., Zhao W. (2015). Inhibitory effects of cytochrome P450 enzymes CYP1A2, CYP2A6, CYP2E1 and CYP3A4 by extracts and alkaloids of Gelsemium elegans roots. J. Ethnopharmacol. 166 66–73. 10.1016/j.jep.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R. (2005). Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 352 2211–2221. 10.1056/NEJMra032424 [DOI] [PubMed] [Google Scholar]
- Xiong X., Peng W., Chen L., Liu H., Huang W., Yang B., et al. (2015). Traditional Chinese medicine Zhiqiao–Houpu herb-pair induce bidirectional effects on gastric motility in rats. J. Ethnopharmacol. 175 444–450. 10.1016/j.jep.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Yin J., Luo Y., Deng H., Qin S., Tang W., Zeng L., et al. (2014). Hugan Qingzhi medication ameliorates hepatic steatosis by activating AMPK and PPARα pathways in L02 cells and HepG2 cells. J. Ethnopharmacol. 154 229–239. 10.1016/j.jep.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Yoshida N., Takagi A., Kitazawa H., Kawakami J., Adachi I. (2005). Inhibition of P-glycoprotein-mediated transport by extracts of and monoterpenoids contained in zanthoxyli fructus. Toxicol. Appl. Pharmacol. 209 167–173. 10.1016/j.taap.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Zhang Y. J., Huang W., Huang X., Wang Y., Wang Z., Wang C., et al. (2012). Fructus aurantii induced antidepressant effect via its monoaminergic mechanism and prokinetic action in rat. Phytomedicine 19 1101–1107. 10.1016/j.phymed.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Zhou D. Y., Chen D. L., Xu Q., Xue X. Y., Zhang F. F., Liang X. M. (2007). Characterization of polymethoxylated flavones in Fructus aurantii by liquid chromatography with atmospheric pressure chemical ionization combined with tandem mass spectrometry. J. Pharm. Biomed. Anal. 43 1692–1699. 10.1016/j.jpba.2006.12.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data are contained within the manuscript.


