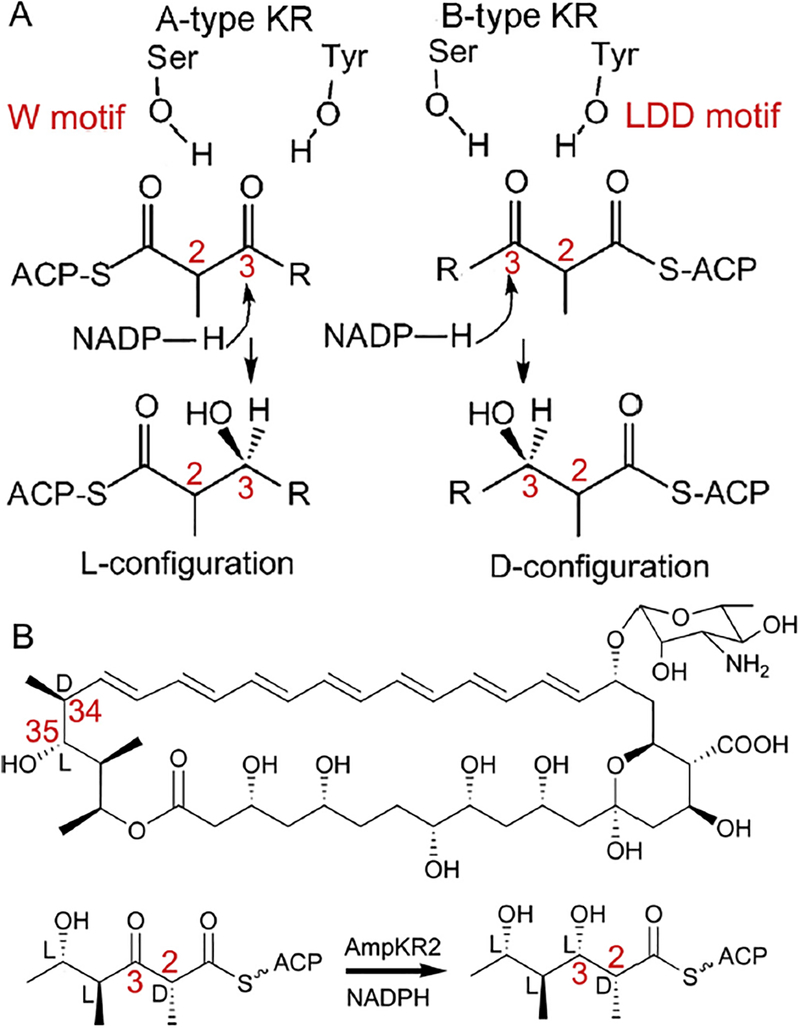
Fig. 1.
The proposed mechanism for control of the stereochemistry of C3 hydroxyl substituents by a KR domain. (A) The W motif of A-type KRs and the LDD motif of B-type KRs are hypothesized to direct the substrate to enter the active site from opposite sides. (B) AmpKR2 in Amphotericin biosynthesis. AmpKR2 reduces the C3 keto group of (2D, 4L, 5L)-5-hydroxy-2,4-dimethyl-3-oxohexanoyl-AmpACP2 to an L-hydroxyl and preserves the D-orientation of the C2-methyl group.