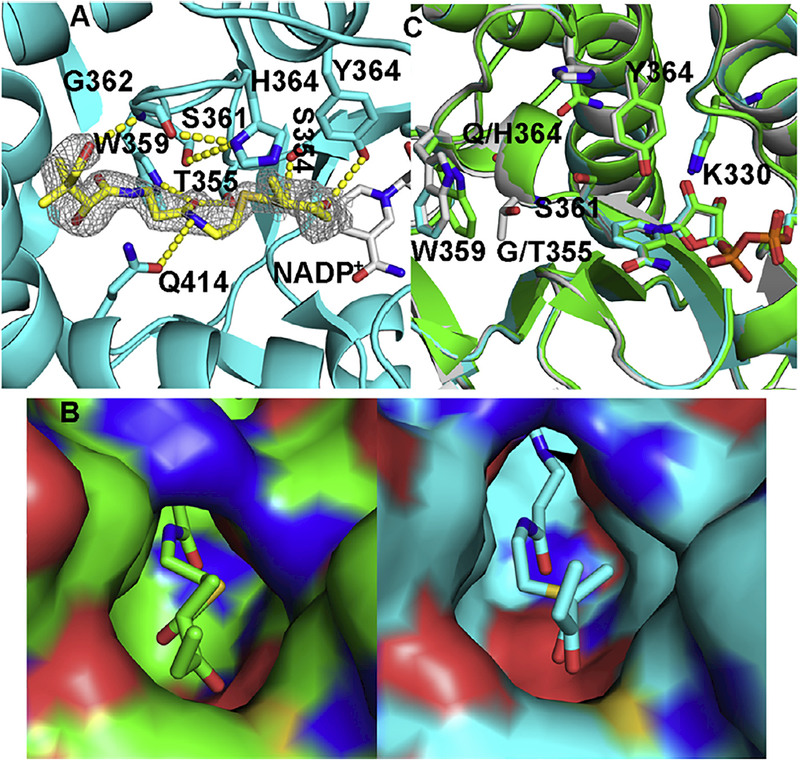
Fig. 3.
Ternary complex of AmpKR2 G355T/Q364H mutant. (A) Hydrogen bonds between the substrate and the active site of the AmpKR2 G355T/Q364H mutant. The |Fo| – |Fc| omit map of the substrate was superimposed on the final model of the ternary complex of AmpKR2 G355T/Q364H mutant and contoured at 2.5 σ. (B) The 2-methyl-3-oxopentanoyl binding pocket of wild type AmpKR2 (green) and the G355T/Q364H mutant (cyan). (C) Structural comparison of ternary structure of wild type AmpKR2 (green), the ternary structure of the G335T/Q364H mutant (cyan), and the binary structure of the G335T/Q364H mutant (grey).