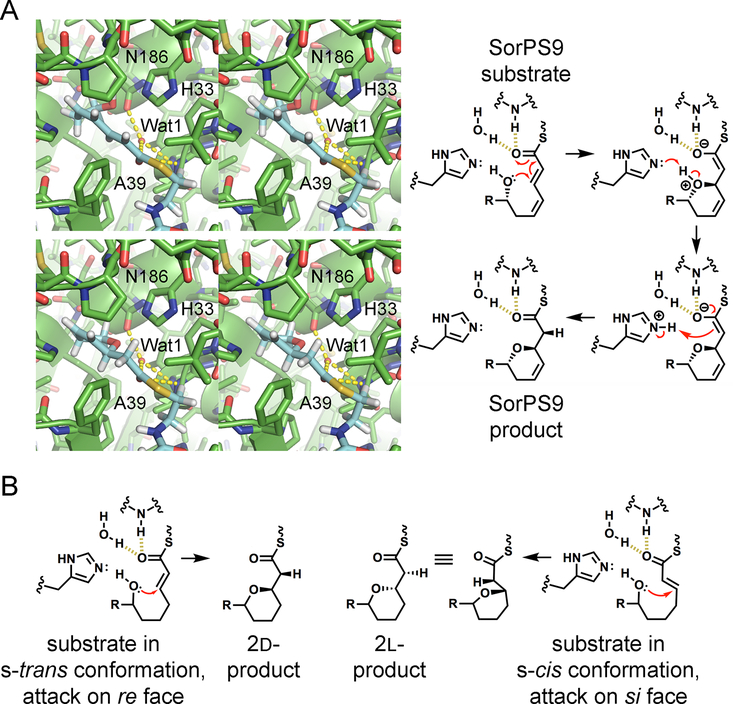
Figure 5.
Proposed mechanism of ring formation by PSs. (A) Stereodiagrams show relevant portions of the SorPS9 substrate and product modeled in the SorPS9 active site. The thioester carbonyl was positioned next to Wat1 and the NH of A39, as observed in the PpsDH4 complex structure (PDB entry 5NJI), in a putative oxyanion hole.24 This would facilitate a conjugate addition of the hydroxyl group to Cβ. The catalytic histidine H33 is in position to mediate proton transfer to Cα. Whether N186 forms a hydrogen bond with the substrate is not clear; it may interact with only Wat1. As mentioned in Figure 1, the actual SorPS9 substrate may also possess a D-ε-hydroxyl group. (B) For an attack at the re face, like that mediated by SorPS9, the bond between the thioester carbonyl and Cα must be in the s-trans conformation. An attack at the si face may be enabled by positioning a substrate so that this bond is in the s-cis conformation.