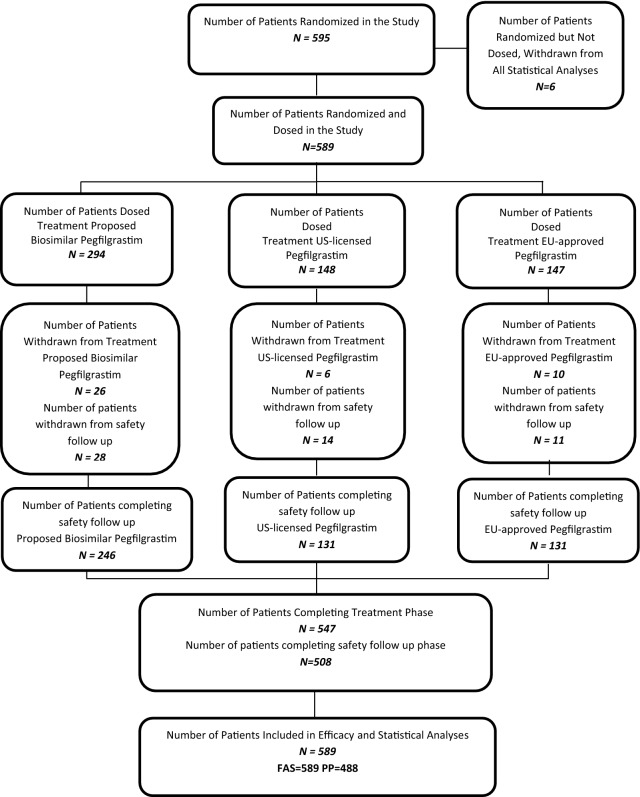
Fig. 1.
Disposition of patients as randomized: distribution of randomized patients into three arms (the proposed pegfilgrastim biosimilar, US-licensed pegfilgrastim reference product, an EU-approved pegfilgrastim reference product), including the number of patients that withdrew and completed treatment and safety follow up