Abstract
Purpose
Dose-volume histogram (DVH) toxicity relationships are poorly defined in men who receive radiation after radical prostatectomy (RP). We evaluated Radiation Therapy Oncology Group (RTOG) study 0534 and institutional intact normal-tissue sparing guidelines, as well as dose to bladder trigone, for ability to minimize late toxicity.
Methods and materials
164 men received intensity modulated radiation therapy (RT) to a median prostate bed dose of 66.6 Gy at a median of 22 months after RP. 46% of men were prescribed androgen deprivation therapy and pelvic lymph node irradiation to a median dose of 50.4 Gy. DVH relationships for the rectum, bladder, trigone, and bladder excluding the clinical target volume (bladder-CTV) were analyzed against the Common Terminology Criteria for Adverse Events late grade 2 + (G2+) gastrointestinal (GI) and genitourinary (GU) toxicity by log-rank test. RTOG 0534 (rectum V65, 40 Gy ≤35, 55%, and bladder-CTV V65, 40 ≤50, 70%) and intact prostate RT institutional guidelines (rectum V70, 65, 40 ≤20, 40, 80% and bladder V70, 65, 40 ≤30, 60, 80%, respectively) guidelines were evaluated.
Results
With a median follow-up time of of 33 months, the 4-year freedom from G2 + GI and GU toxicity were both 91%. G2 + GI (n = 12) and GU (n = 15) toxicity included 4% diarrhea (n = 6), 4% hemorrhage (n = 6), 1% proctitis (n = 1), and 4% urinary frequency (n = 7), 1% obstructive (n = 2), 2% cystitis (n = 3), and 3% incontinence (n = 5), respectively. RTOG 0534 rectum and bladder goals were not achieved in 65% and 41% of cases, while the institutional intact prostate goals were not achieved in 21% and 25% of cases, respectively. Neither dose to the bladder trigone nor any of the proposed normal tissue goals were associated with late toxicity (P > .1). In the univariate analysis, age, pelvic RT, RT dose, anticoagulation use, androgen deprivation therapy, time from RP to RT, and tobacco history were not associated with toxicity.
Conclusions
More than 90% of men were free from late G2 + toxicity 4 years after post-RP intensity modulated RT. No tested parameters were associated with late toxicity. In the absence of established normal-tissue DVH guidelines in the postoperative setting, the use of intact guidelines is reasonable.
Summary.
Dose-volume histogram toxicity relationships are poorly defined in men who receive radiation after radical prostatectomy. We evaluated Radiation Therapy Oncology Group study 0534 and institutional intact normal-tissue sparing guidelines as well as dose to bladder trigone for ability to minimize late toxicity. More than 90% of men were free from late G2 + toxicity 4 years after postradical prostatectomy intensity modulated radiation therapy and no tested parameters were associated with late toxicity. The use of intact guidelines may be reasonable in the postoperative setting.
Alt-text: Unlabelled box
Introduction
The minimization of late treatment-related morbidity is highly prioritized in men who receive post-prostatectomy prostate radiation therapy (PPRT) because of their longevity with ≥74% of patients expected to live at least 10 years after either adjuvant or salvage radiation therapy.1, 2, 3, 4, 5 Furthermore, most men with prostate cancer will die from competing risks.6 Despite evidence of improvements in disease control2, 3, 5, 7 and in some patients survival,1, 4 adjuvant PPRT remains underutilized,8, 9, 10 likely due in part to the perceived risk of late toxicity.
These concerns could be mitigated by evidence-based normal-tissue sparing dose constraints for PPRT. However, data that establish dose-volume histogram (DVH) toxicity relationships in the post-prostatectomy setting are sparse. The Radiation Therapy Oncology Group (RTOG) has proposed normal- tissue sparing goals in the protocol for RTOG 0534 (clinicaltrials.gov NCT00567580) but these can be difficult to satisfy because of the large volume of bladder tissue that is included in the prostate bed target volume.11 Moreover, the clinical consequences of failing to meet RTOG goals have not been assessed and critical organ dose constraints may not be adequately captured by RTOG parameters. Doses to the bladder trigone, for example, are likely underestimated by the RTOG bladder clinical target volume (CTV) parameters due to overlapping CTV and trigone volumes. However, maximum trigone doses have been strongly associated with late genitourinary (GU) toxicity in the intact prostate cancer setting.12 The absence of data on post-prostatectomy DVH relationships that are associated with late GU and gastrointestinal (GI) toxicity constitutes a critical knowledge gap that prevents radiation oncologists from minimizing the risks of PPRT.
To address this knowledge gap, we reviewed our experience with PPRT and compared our institutional normal tissue-sparing dose constraints to those specified by RTOG 0534. Our primary goal was to assess the ability of both commonly used sets of DVH guidelines to predict late GU and GI toxicity. Our secondary goal was an attempt to improve these guidelines by evaluating the association between dose to the bladder trigone and late GU toxicity.
Methods and materials
Patients
All men who received PPRT between 2001 and 2012 as part of salvage or adjuvant therapy were identified from an internal review board-approved clinical database at a single academic institution. Patient records were prospectively collected.
Treatment
Computed tomography-based simulation and radiation therapy planning were in accordance with recommendations published by the American College of Radiology.13 All men were treated with intensity modulated radiation therapy (IMRT). Prostate bed volumes were contoured according to anatomy-based interdisciplinary consensus guidelines,14 with a 0.5 to 1 cm planning target volume margin. A subset of patients received pelvic field radiation therapy and androgen deprivation therapy (ADT) per the institutional guidelines, which generally included patients with a higher Gleason score, younger age, or lymph node positivity.
The pelvic field was defined using a standard CTV margin of 7 mm to 8 mm around pelvic blood vessels with an additional 7 mm to 8 mm planning target volume expansion. Prior to 2007, image guidance consisted of alignment to bony anatomy but after 2007, cone beam computed tomography was used to evaluate soft-tissue alignment of the prostate bed. ADT consisted of dual-agent therapy with an antiandrogen and a luteinizing hormone-releasing hormone agonist. ADT was generally given for a duration of 4 months, beginning 2 months prior to radiation therapy.
Dose-volume histogram analysis
For normal-tissue DVH analysis, the rectum was contoured from the ischial tuberosities to the rectosigmoid junction.15 The bladder was defined by its outer wall. The trigone was retrospectively contoured on archived image sets as the posterior triangular portion of the bladder between the vesicoureteral junctions superiorly and the vesicourethral junction inferiorly.12 DVH parameters were analyzed in association with the maximal Common Toxicity Criteria (CTC) version 3.0 toxicity grade. DVH parameters for the rectum, bladder, trigone, and bladder excluding the clinical target volume (bladder-CTV) were evaluated for compliance with all guidelines from RTOG 0534 (rectum V65, 40 Gy ≤35, 55%, and bladder-CTV V65, 40 ≤50, 70%, respectively) and institutional guidelines (rectum V70, 65, 40 ≤20, 40, 80% and bladder V70, 65, 40 ≤30, 60, 80%, respectively; Table 1). The institutional dose constraints were initially proposed in 2007 to limit morbidity for men with intact prostate cancer who received external beam radiation therapy.16
Table 1.
RTOG versus institutional dose constraints
| RTOG | Institutional | ||
|---|---|---|---|
| Rectum | Rectum | V70 Gy ≤ 20% | |
| V65 Gy ≤ 35% | V65 Gy ≤ 40% | ||
| V40 Gy ≤ 55% | V40 Gy ≤ 80% | ||
| Bladder-CTV | Bladder | V70 Gy ≤ 30% | |
| V65 Gy ≤ 50% | V65 Gy ≤ 60% | ||
| V40 Gy ≤ 70% | V40 Gy ≤ 80% | ||
CTV, clinical target volume; RTOG, Radiation Therapy Oncology Group.
Follow up
Patients were seen 4 to 6 weeks after therapy and then every 6 months for the first 2 years. After 2 years, patients were seen every 6 to 9 months until 5 years after therapy, after which they were seen annually. Both GU and GI systems were evaluated at each follow-up visit. RTOG and CTC toxicity grades were recorded prospectively during follow up after 2007. Patients who completed treatment prior to 2007 were retrospectively assigned CTC toxicity grades. Toxicities were graded relative to baseline pre-radiation therapy function. Late toxicities were defined as those that occurred >3 months after radiation therapy. For the purposes of this study, only late CTC toxicity grades were analyzed.
Statistical analysis
Freedom from GI and GU toxicities was estimated using the Kaplan-Meier method. Follow-up time was calculated from the end of radiation therapy. Any single event that met the criteria for the toxicity grade that was analyzed would permanently drop the freedom-from-toxicity curve. Several clinical, disease, and treatment factors were tested against late GU and GI toxicities in the univariate analysis using the log-rank test. These factors included age, pelvic radiation therapy, radiation therapy dose, anticoagulation use, ADT, time from radical prostatectomy (RP) to radiation therapy, tobacco history, and whether RTOG and/or institutional guidelines were met. All statistical analysis was performed with JMP13 (SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 164 men were identified as eligible for this study. Five patients were excluded due to no available toxicity data, which yielded a total of 159 analyzable cases. The median age was 61 years. The median pre-RP prostate-specific antigen level was 7. Most men had pT3N0 disease and Gleason score ≥7. Detailed patient characteristics are presented in Table 2.
Table 2.
Patient and treatment characteristics (n = 159)
| Number (%) or Median (IQR) | |
|---|---|
| Age (years) | 61 (Range, 47-80) |
| T stage | |
| pT2 | 48 (30%) |
| pT3a | 74 (47%) |
| pT3b | 29 (18%) |
| N stage | |
| pN0 | 128 (80%) |
| pN1 | 13 (8%) |
| pNx | 23 (15%) |
| Patholgic Gleason score | |
| 2-6 | 23 (15%) |
| 7 | 94 (59%) |
| 8-10 | 42 (26%) |
| Pre-radical prostatectomy prostate-specific antigen level (ng/mL) | 7 (5-12.3) |
| Radiation dose (Gy) | 66.6 (66-68.4) |
| Pelvic lymph node radiation therapy | 73 (46%) |
| Concurrent androgen deprivation therapy | 73 (46%) 4 (Range, 3-28) |
| Duration (mo) | |
| Follow-up time (mo) | 33 (18-59) |
IQR, interquartile range.
Treatment parameters
The median time from RP to radiation therapy was 22 months, which was given as adjuvant (13%) or salvage (87%) therapy. The median dose to the prostate bed was 66.6 Gy (interquartile range [IQR], 66-68.4). Seventy-three men (46%) received both pelvic lymph node irradiation (median dose 50.4 Gy) and concurrent ADT (median duration 4 months).
Characterization of late toxicity
The median follow-up time was 33 months from the end of radiation therapy to the last follow up. Freedom from grade 2 or higher (G2+) GI and GU toxicity at 4 years were both 91%. G2 + GI (n = 12) and GU (n = 15) toxicity included 4% diarrhea (n = 6), 4% hemorrhage (n = 6), and 1% proctitis (n = 1), and 4% urinary frequency (n = 7), 1% obstructive (n = 2), 2% cystitis (n = 3), and 3% incontinence (n = 5), respectively. Two patients had G3 + GU toxicity and no patients had G3 + GI toxicity.
Dose-volume histogram analysis and univariate analysis of factors that are associated with late toxicity
A total of 141 of 159 patients (89%) had complete DVH data available for analysis. A previously used treatment planning software was no longer accessible for the remaining patients. A total of 89 men had simulation image sets available in which the trigone could be contoured and the CTV could be subtracted from the bladder with dose recalculations. The median volume of bladder and bladder-CTV were 180 cc and 154 cc, respectively. The trigone median volume was 16.4 cc (IQR, 12.5-20.6 cc; Range, 6-35 cc).
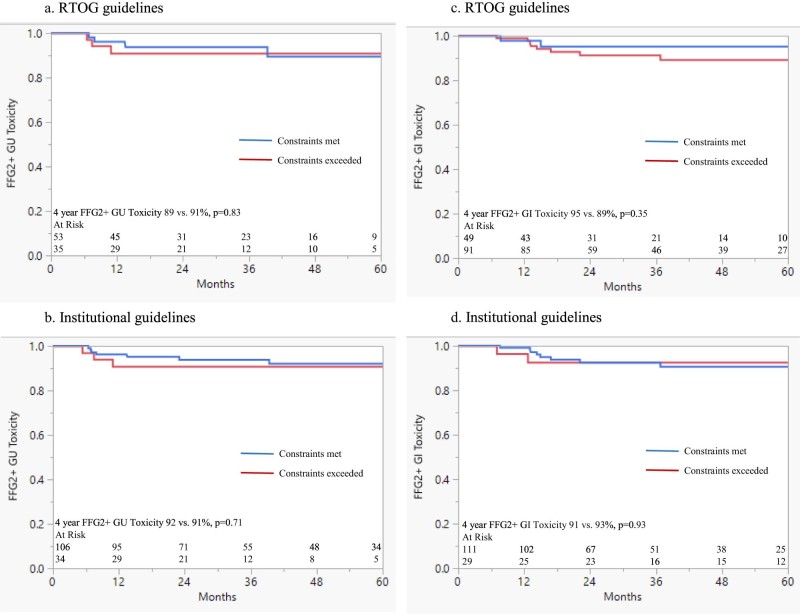
RTOG 0534 rectum and bladder goals were not achieved in 65% and 41% of cases, respectively. If the bladder-CTV volume was greater than the median, RTOG bladder dose constraints were more likely to be met (66% vs. 49%; P = .09). The institutional goals were not achieved in 21% of rectum and 25% of bladder cases, respectively. Neither the RTOG nor the institutional proposed goals were significantly associated with late toxicity (Fig 1A-D). The median bladder trigone V70 Gy was 23%. Dose to the bladder trigone was not associated with GU toxicity (Table 3). Dose to the bladder-CTV was not associated with GU toxicity at various dose levels (Table 4). The univariate analysis for age, pelvic radiation therapy, radiation therapy dose, anticoagulation use, ADT, time from RP to radiation therapy, and tobacco history were not associated with toxicity (Table 5).
Figure 1.
Freedom from grade ≥2 (FFG2+) Common Toxicity Criteria toxicity stratified by whether (A) Radiation Therapy Oncology Group bladder dose constraints were met (n = 89), (B) institutional bladder dose constraints were met (n = 141), (C) Radiation Therapy Oncology Group rectal dose constraints were met (n = 141), or (D) institutional rectal dose constraints were met (n = 141).
Table 3.
Four-year freedom from grade ≥2 genitourinary toxicity stratified by dose to trigone (n = 89)
| Genitourinary toxicity | |
|---|---|
| V70 Gy < 30 (vs. ≥30) | 91% vs. 89%, P = .49 |
| V70 Gy < 23% (median) | 93% vs. 87%, P = .17 |
| V70 Gy < 20% | 93% vs. 88%, P = .24 |
| V70 Gy < 15% | 92% vs. 89%, P = .35 |
| V70 Gy < 10% | 92% vs. 90%, P = .46 |
| V70 Gy < 5% | 91% vs. 90%, P = .58 |
Table 4.
Four-year freedom from grade ≥2 genitourinary toxicity stratified by dose to median volume of bladder-clinical target volume (n = 89)
| Genitourinary Toxicity | |
|---|---|
| V70 Gy < 5% (vs. ≥5) | 93% vs. 88%, P = .21 |
| V65 Gy < 27% | 90% vs. 90%, P = .62 |
| V60 Gy < 37% | 91% vs. 89%, P = .52 |
| V50 Gy < 50% | 91% vs. 90%, P = .61 |
Table 5.
Freedom from grade ≥2 gastrointestinal and genitourinary toxicity stratified by clinical parameters
| Freedom from grade ≥2 toxicity (P-value) |
||
|---|---|---|
| Gastrointestinal | Genitourinary | |
| Tobacco (history vs. never; n = 154) | .58 | .39 |
| Time from radical prostatectomy to radiation therapy >22 mo (median; yes vs. no; n = 158) | .20 | .99 |
| Anticoagulant use (yes vs. no; n = 96) | .99 | .36 |
| Age >60 years (median; yes vs. no; n = 158) | .28 | .77 |
| Radiation therapy dose >66 Gy (median; yes or no; n = 158) | .09 | .85 |
| Pelvic lymph node radiation therapy (yes vs. no; n = 158) | .48 | .39 |
| Androgen deprivation therapy use (yes vs. no; n = 158) | .89 | .48 |
| Prostate bed volume >91 cc (median; yes or no; n = 89) | .87 | .83 |
| Rectal volume >92 cc (median; yes vs. no; n = 140) | .47 | N/A |
| Bladder volume >180 cc (median; yes vs. no; n = 140) | N/A | .95 |
N/A, non-applicable.
Discussion
In this study, we describe the late toxicities of post-RP IMRT and investigated DVH-toxicity relationships. We found low rates of long-term morbidity with a 4-year freedom from G2 + toxicity of 91% in both the GI and GU domains. No tested DVH parameter threshold was significantly associated with late toxicity, although the analyses were likely underpowered due to the low event rate. Patient and treatment factors that were tested in this study were not associated with toxicity including age, anticoagulation use, ADT, time from RP to radiation therapy, tobacco history, and compliance with RTOG and/or institutional dose constraints.
Our report that >90% of men who received post-RP IMRT were free from G2 + GI and GU toxicity at 4 years contributes to the growing evidence that modern PPRT has a low risk of significant morbidity. Retrospective studies of post-RP IMRT have reported 2-year CTC late G2 + GI and GU toxicity rates of 0% to 2% and 12% to 17%, respectively.17, 18, 19 A recent prospective phase 2 study of 68 men who were treated with guideline-based post-RP IMRT reported cumulative 5-year CTC late G2 GI and GU toxicity incidences of 12.3% and 10.6%, respectively, with no G3 + toxicities.20 These and our results compare favorably with the RTOG late G2 + GU toxicity of 21.3% that was reported in European Organisation for Research and Treatment of Cancer study 22911 and the 23.8% complication rate that was reported in Southwest Oncology Group study 8794 (both studies used conventional radiation therapy techniques).2, 7 Several studies also demonstrated no significant long-term changes in patient-reported quality of life after PPRT.21, 22, 23 Collectively, these findings should alleviate any concerns of long-term PPRT toxicity that may lead to underutilization.
To maximally exploit the advantages of IMRT, DVH-toxicity relationships of critical organs must be well defined. Normal-tissue complication probability modeling predicts that late G3 + GI and GU toxicities increase by 1.2% and 0.7%, respectively, per 1 Gy increase in prescription dose with an upward inflection point in the 68 Gy to 70 Gy range,24 which suggests that critical organ dose thresholds should exist. Indeed, a retrospective study of 128 men who received conventional or 3-dimensional conformal radiation therapy found that rectal V50 ≥63% and acute GI bleeding were independently predictive of late GI bleeding in the multivariate analysis.25 However, this rectal DVH constraint is more permissive than the RTOG goal of rectum V40 ≤55% and its relevance to post-RP IMRT remains unclear. In the prospective trial of guideline-based post-RP IMRT that reported low rates of late G2 toxicity, 97% of patients met planning constraints of rectal wall D1cc ≤66 Gy, posterior rectal wall D1cc ≤55 Gy, and bladder wall D2cc ≤67.3 Gy.20 However, both the rectum and bladder were contoured as hollow organs with 3 mm thick walls, which complicated comparisons to RTOG and other institutional guidelines.
To our knowledge, our analysis is the first to evaluate the association of RTOG PPRT DVH goals with late toxicity. The absence of significant associations between late G2 + toxicity and the assessed DVH constraints suggests that failing to satisfy RTOG normal-tissue sparing goals has limited long-term clinical consequences. Our results are consistent with a recent study that demonstrated no change in late toxicity after RTOG guideline adoption despite significant increases in prostate bed volumes and GU DVH parameters.11 Until stronger evidence of DVH-toxicity relationships is available from RTOG 0534 and other randomized trials,26, 27 the adoption of dose constraints as established in the intact setting makes sense, which in our analyses was no less useful than RTOG 0534 guidelines. Although dose constraints in the relatively lower dose of the postoperative setting should conceptually be more conservative than those for the intact setting to achieve a biologically equivalent effect on normal tissues, maintaining consistent guidelines between the intact and postoperative settings is useful, particularly in the absence of any known discriminating metrics between both settings.
We did not observe a relationship between dose to the bladder or bladder trigone and late GU toxicity in the current study. However, previous data have demonstrated an association between bladder V70 and patient-reported urinary incontinence after PPRT.22 Furthermore, the bladder trigone has been proposed to play roles in both bladder filling and micturition, which suggests a plausible mechanism for urinary side effects from high-dose radiation-mediated disruption of trigone physiology.28, 29 In a dosimetric study of IMRT for intact prostate cancer, the maximum dose to the trigone predicted large post-treatment increases in urinary symptoms and trigone V90 was associated with late G2 + GU toxicity.12 Therefore, further research to investigate the effects of minimizing trigone dose on late GU toxicity is warranted.
Prior studies have identified patient and treatment factors other than normal-tissue doses that modulate late toxicity risk. Specifically, older age and anticoagulation use have been associated with increased late GI toxicity,30 and tobacco use has been associated with increased late GU toxicity.31 Although adjuvant ADT has been reported to decrease rectal radiation tolerance,32 recent level 1 evidence revealed no increased late toxicity from 6 months of concurrent and adjuvant ADT compared with salvage post-RP radiation therapy alone.33 The association of radiation therapy timing after RP on urinary continence is controversial.34, 35, 36 Finally, although diabetes mellitus has been shown to increase the risk of late GU toxicity, data with regard to its impact on late GI toxicity are conflicting.37, 38 Our analysis revealed no significant association of late GI or GU toxicity with age, anticoagulation use, tobacco history, ADT, or time from RP to radiation therapy. The absence of these associations may be attributed in part to the lower radiation doses that are prescribed in the post-RP setting.
This study bears the limitations of its retrospective design. RTOG toxicity grades were recorded prospectively for all patients but CTC grades were retrospectively assigned prior to 2007 and thus potentially biased in some cases. Although our cohort of 164 men is comparable with or larger than those in other published studies of post-RP IMRT,18, 19, 20 the low rates of late G2 + GI and GU toxicities suggest that much larger cohorts may be required to sufficiently evaluate DVH-toxicity relationships.
The narrow range of prescription doses (IQR, 66-68.4 Gy) represented in our cohort also limited our ability to investigate high-dose effects on normal tissues. Finally, the median follow-up time of 33 months may not have been sufficient to capture late GU toxicity, which—unlike GI toxicity—may increase even >2 years after radiation therapy.39 With a longer follow-up period, DVH-toxicity relationships in the GU domain may have become significant. Despite these limitations, our study provides useful clinical information to evaluate the risk of late toxicities from PPRT while we wait for the results of large prospective data studies such as RTOG 0534.
In our practice, we continue to prescribe 64 Gy for adjuvant PPRT and 68 Gy to 68.4 Gy for standard salvage PPRT while using absolute constraints (rectum V70, 65, 40 ≤20, 40, 80% and bladder V70, 65, 40 ≤30, 60, 80%, respectively) according to our institutional intact prostate cancer guidelines.
With advances in delivery such as volumetric modulated arc therapy, stricter constraints for intact prostate cancer (rectum V70, 65, 40 ≤10, 20, 40% and bladder V70, 65, 40 ≤15, 30, 60% respectively) are the initial planning goal and often achievable in the postoperative setting. In men with a gross local recurrence, we consider a simultaneous integrated boost to deliver ≥70 Gy to the recurrent disease but also evaluate the normal tissue V70 (particularly in the bladder trigone) to potentially reduce the risk of late GU toxicity.
Conclusions
Post-RP IMRT resulted in low rates of morbidity with >90% of men free from late G2 + toxicity in the GI and GU domains at 4 years. Neither RTOG 0534 nor institutional guidelines were significantly associated with late toxicity. In the absence of well-established DVH-toxicity relationships, the use of intact prostate-cancer normal-tissue sparing goals for post-RP IMRT is reasonable. Risk factors that increase late morbidity after dose-escalated radiation therapy for intact prostate cancer may not be relevant for men who receive lower adjuvant or salvage radiation doses.
Footnotes
Meeting information: The preliminary data was presented at the 2015 Annual Meeting of the American Society for Radiation Oncology in San Antonio, Texas from October 18 to 21, 2015.
Sources of support: No financial support was received for this project.
Conflicts of Interest: None of the authors have conflict of interest disclosures.
References
- 1.Thompson I.M., Tangen C.M., Paradelo J. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolla M., van Poppel H., Tombal B. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 3.Wiegel T., Bartkowiak D., Bottke D. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–250. doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Trock B.J., Han M., Freedland S.J. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shipley W., Seiferheld W., Lukka H. Report of NRG Oncology/RTOG 9601, A phase 3 trial in prostate cancer: Anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) in patients following radical prostatectomy (RP) with pT2-3pN0 disease and an elevated PSA. Int J Radiat Oncol Biol Phys. 2016;94:3–6. [Google Scholar]
- 6.Zaorsky N.G., Churilla T.M., Egleston B.L. Causes of death among cancer patients. Ann Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson I.M., Jr, Tangen C.M., Paradelo J. Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 8.Maurice M.J., Zhu H., Abouassaly R. Low use of immediate and delayed postoperative radiation for prostate cancer with adverse pathological features. J Urol. 2015;194:972–976. doi: 10.1016/j.juro.2015.03.122. [DOI] [PubMed] [Google Scholar]
- 9.Mahal B.A., Hoffman K.E., Efstathiou J.A., Nguyen P.L. National trends in the recommendation of radiotherapy after prostatectomy for prostate cancer before and after the reporting of a survival benefit in March 2009. Clin Genitourin Cancer. 2015;13:e167–e172. doi: 10.1016/j.clgc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Sineshaw H.M., Gray P.J., Efstathiou J.A., Jemal A. Declining use of radiotherapy for adverse features after radical prostatectomy: Results from the National Cancer Data Base. Eur Urol. 2015;68:768–774. doi: 10.1016/j.eururo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Marcus D.M., Rossi P.J., Cooper S., Jani A.B. Adoption of Radiation Therapy Oncology Group consensus guidelines for post-prostatectomy radiation therapy in an academic tertiary care center. Radiat Oncol. 2014;3:395–400. [Google Scholar]
- 12.Ghadjar P., Zelefsky M.J., Spratt D.E. Impact of dose to the bladder trigone on long-term urinary function after high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:339–344. doi: 10.1016/j.ijrobp.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaorsky N.G., Showalter T.N., Ezzell G.A. ACR Appropriateness Criteria® external beam radiation therapy treatment planning for clinically localized prostate cancer, part I of II. Adv Radiat Oncol. 2017;2:62–84. doi: 10.1016/j.adro.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiltshire K.L., Brock K.K., Haider M.A. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;69:1090–1099. doi: 10.1016/j.ijrobp.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 15.Gay H.A., Barthold J.H., O'Meara E. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pederson A.W., Fricano J., Correa D. Late toxicity after intensity-modulated radiation therapy for localized prostate cancer: an exploration of dose-volume histogram parameters to limit genitourinary and gastrointestinal toxicity. Int J Radiat Oncol Biol Phys. 2012;82:235–241. doi: 10.1016/j.ijrobp.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 17.Hunter G.K., Brockway K., Reddy C.A. Late toxicity after intensity modulated and image guided radiation therapy for localized prostate cancer and post-prostatectomy patients. Pract Radiat Oncol. 2013;3:323–328. doi: 10.1016/j.prro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Nath S.K., Sandhu A.P., Rose B.S. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:435–441. doi: 10.1016/j.ijrobp.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Goenka A., Magsanoc J.M., Pei X. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol. 2011;60:1142–1148. doi: 10.1016/j.eururo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Berlin A., Cho E., Kong V. Phase 2 trial of guideline-based postoperative image guided intensity modulated radiation therapy for prostate cancer: Toxicity, biochemical, and patient-reported health-related quality-of-life outcomes. Pract Radiat Oncol. 2015;5:e473–e482. doi: 10.1016/j.prro.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Melotek J.M., Liao C., Liauw S.L. Quality of life after post-prostatectomy intensity modulated radiation therapy: Pelvic nodal irradiation is not associated with worse bladder, bowel, or sexual outcomes. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0141639. e0141639.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son C.H., Melotek J.M., Liao C. Bladder dose-volume parameters are associated with urinary incontinence after postoperative intensity modulated radiation therapy for prostate cancer. Pract Radiat Oncol. 2015;6:e179–e185. doi: 10.1016/j.prro.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Johnson M.E., Zaorsky N.G., Martin J.M. Patient reported outcomes among treatment modalities for prostate cancer. Can J Urol. 2016;23:8535–8545. [PubMed] [Google Scholar]
- 24.Ohri N., Dicker A.P., Trabulsi E.J., Showalter T.N. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer. 2012;48:837–844. doi: 10.1016/j.ejca.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozzarini C., Fiorino C., Ceresoli G.L. Significant correlation between rectal DVH and late bleeding in patients treated after radical prostatectomy with conformal or conventional radiotherapy (66.6-70.2 Gy) Int J Radiat Oncol Biol Phys. 2003;55:688–694. doi: 10.1016/s0360-3016(02)04117-2. [DOI] [PubMed] [Google Scholar]
- 26.Ghadjar P., Hayoz S., Bernhard J. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: First results of the randomized trial SAKK 09/10. J Clin Oncol. 2015;33:4158–4166. doi: 10.1200/JCO.2015.63.3529. [DOI] [PubMed] [Google Scholar]
- 27.Parker C., Clarke N., Logue J. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) Clin Oncol (R Coll Radiol) 2007;19:167–171. doi: 10.1016/j.clon.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Roosen A., Wu C., Sui G., Chowdhury R.A., Patel P.M., Fry C.H. Characteristics of spontaneous activity in the bladder trigone. Eur Urol. 2009;56:346–353. doi: 10.1016/j.eururo.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Marks L.B., Carroll P.R., Dugan T.C., Anscher M.S. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257–1280. doi: 10.1016/0360-3016(94)00431-J. [DOI] [PubMed] [Google Scholar]
- 30.Choe K.S., Jani A.B., Liauw S.L. External beam radiotherapy for prostate cancer patients on anticoagulation therapy: How significant is the bleeding toxicity? Int J Radiat Oncol Biol Phys. 2010;76:755–760. doi: 10.1016/j.ijrobp.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Solanki A.A., Liauw S.L. Tobacco use and external beam radiation therapy for prostate cancer: Influence on biochemical control and late toxicity. Cancer. 2013;119:2807–2814. doi: 10.1002/cncr.28128. [DOI] [PubMed] [Google Scholar]
- 32.Sanguineti G., Agostinelli S., Foppiano F. Adjuvant androgen deprivation impacts late rectal toxicity after conformal radiotherapy of prostate carcinoma. Br J Cancer. 2002;86:1843–1847. doi: 10.1038/sj.bjc.6600266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrie C., Hasbini A., de Laroche G. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–756. doi: 10.1016/S1470-2045(16)00111-X. [DOI] [PubMed] [Google Scholar]
- 34.Sowerby R.J., Gani J., Yim H., Radomski S.B., Catton C. Long-term complications in men who have early or late radiotherapy after radical prostatectomy. Can Urol Assoc J. 2014;8:253–258. doi: 10.5489/cuaj.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petroski R.A., Warlick W.B., Herring J. External beam radiation therapy after radical prostatectomy: Efficacy and impact on urinary continence. Prostate Cancer Prostatic Dis. 2004;7:170–177. doi: 10.1038/sj.pcan.4500718. [DOI] [PubMed] [Google Scholar]
- 36.Suardi N., Gallina A., Lista G. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol. 2014;65:546–551. doi: 10.1016/j.eururo.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Kalakota K., Liauw S.L. Toxicity after external beam radiotherapy for prostate cancer: An analysis of late morbidity in men with diabetes mellitus. Urology. 2013;81:1196–1201. doi: 10.1016/j.urology.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 38.Zaorsky N.G., Shaikh T., Ruth K. Prostate cancer patients with unmanaged diabetes or receiving insulin experience inferior outcomes and toxicities after treatment with radiation therapy. Clin Genitourin Cancer. 2017;15:326–335. doi: 10.1016/j.clgc.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelefsky M.J., Chan H., Hunt M., Yamada Y., Shippy A.M., Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]