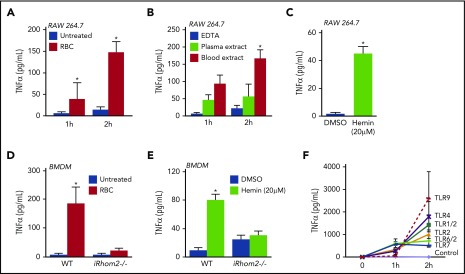
Figure 1.
Coculture of mouse macrophages with fresh blood or blood degradation products. RBCs (4 × 109/mL) (1 hour, 38 ± 38 pg/mL; 2 hours, 146 ± 25 pg/mL) (A) and blood extract from frozen and thawed blood (1 hour, 104 ± 21 pg/mL; 2 hours, 167 ± 23 pg/mL) (B) promoted significantly increased TNF-α shedding from RAW 264.7 cells compared with their controls. (C) Incubation of RAW 264.7 cells with 20 µM hemin also stimulated the release of TNF-α (45 ± 5 pg/mL compared with dimethyl sulfoxide–treated cells, 1 ± 0.8 pg/mL). Incubation with RBC for 4 hours (4 × 109/mL) (D) or with 20 µM hemin for 2 hours (E) stimulated the shedding of TNF-α from WT BMDMs (RBCs, 185 ±56 pg/mL; hemin, 80 ± 8 pg/mL), but not from BMDMs lacking iRhom2 (iRhom2−/−; RBCs, 21 ± 7 pg/mL; hemin, 30 ± 6 pg/mL). (F) Activation of different TLRs on RAW 264.7 cells for 2 hours also triggered the production of TNF-α from macrophages (1424 ± 445 pg/mL for TLR1/2-Pam3CSK4 [0.1 μg/mL]; 1008 ± 191 pg/mL for TLR2, keyhole limpet hemocyanin (108 cells per mL); 1787 ± 671 pg/mL for TLR4, lipopolysaccharide from E. coli (10 ng/mL); 714 ± 278 pg/mL for TLR6/2, FLS-1 (0.5 μg/mL); 506 ± 23 pg/mL for TLR7-ssRNA40 (2.5 μg/mL); 2601 ± 1185 pg/mL for TLR9, ODN1826 [2.5 μg/mL]). The mean ± SEM of 3 independent experiments in triplicate are shown. *P > .05 vs untreated control (A,D), vs EDTA-treated control (B), or vs DMSO-treated control (C,E).