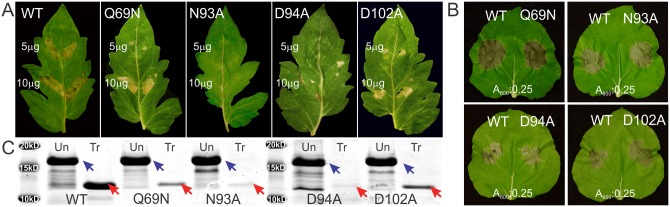
Fig 4. Recognition by the Cf-4 immune receptor and susceptibility to proteolytic degradation of the WT-CfAvr4 and selected ChBD and (GlcNAc)6-binding mutants.
(A) Protein infiltrations of the WT-CfAvr4 (WT) into tomato leaves of cv. Purdue 135 (+Cf-4) induces a strong hypersensitive response (HR) in the infiltrated leaf sectors at both protein concentrations tested. However, CfAvr4 mutants Q69N, D102A only partially trigger an HR and N93A, D94A do not, as seen by the reduced intensity of necrosis. All other ChBD or (GlcNAc)6-binding mutants of CfAvr4 tested (S2 Table) induced the same as the WT-CfAvr4 intensity of HR (S9A Fig). Infiltrations were performed on both the left and right sides of the leaf, and necrosis was evaluated 5 days post-infiltration (dpi). (B) Transient co-expression with Cf-4 of the WT-CfAvr4 (WT) and the Q69N, N93A, D94A and D102A mutants into leaves of Nicotiana benthamiana using an Agrobacterium tumefaciens transient transformation assay, induces, in all cases, a strong and similar in intensity HR. All other mutants are shown in S9B Fig. In all cases, co-infiltrations of Cf-4 with the WT-CfAvr4 were done on the left-hand side of the leaf, whereas co-infiltrations of Cf-4 with the mutant was done on the right-hand side of the leaf. Pictures were taken at 7 dpi. (C) Treatment of the WT-CfAvr4 and Q69N, N93A, D94A, and D102A mutants with 500 ng/μl subtilisin, digests the original full-length protein (blue arrows) to a smaller product that corresponds to the true mature form of CfAvr4 (red arrows). The treatments show that all mutants are vulnerable to proteolytic degradation, as evidenced by the decreased intensity of the mature-form band (red arrows), with mutants N93A and D94A being more susceptible to degradation.