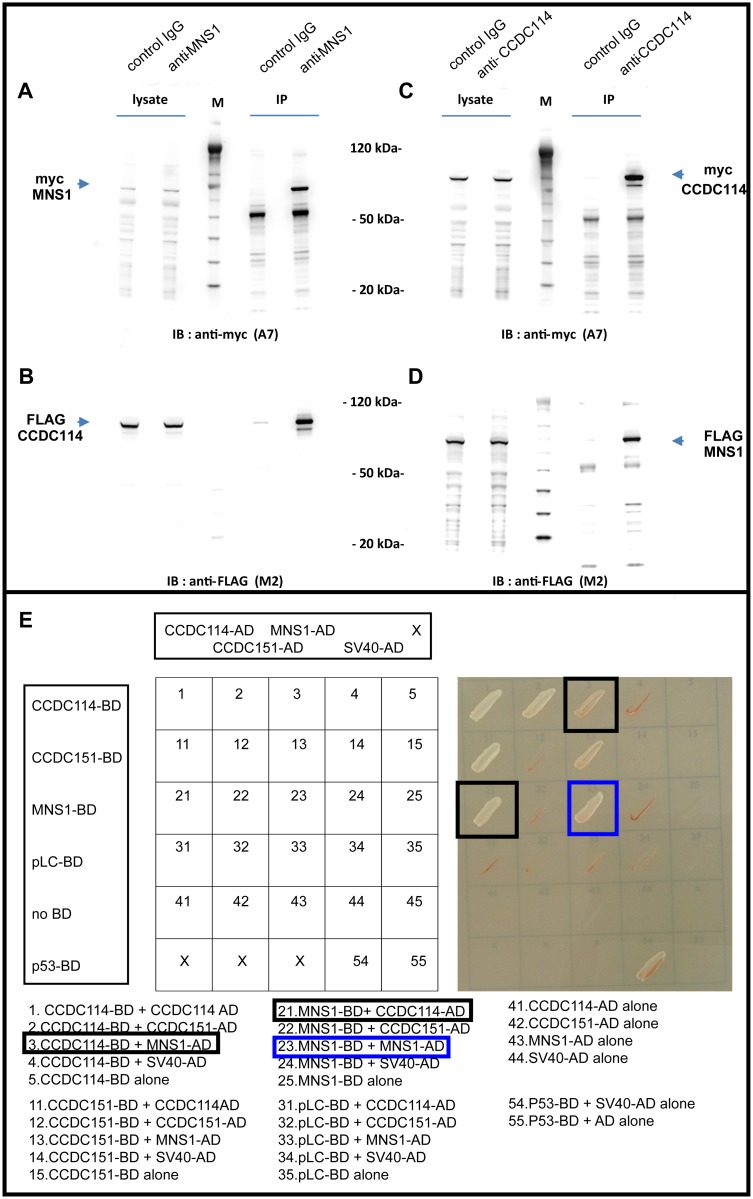
Fig 5. MNS1 forms a dimer and interacts directly with the ODA-DC protein CCDC114.
HEK293 lysates coexpressing FLAG or myc epitope-tagged MNS1 and CCDC114 were immunoprecipitated with either rabbit control IgG and rabbit anti-MNS1 or anti-CCDC114 antibody. Western blotting with mouse anti-myc or anti-FLAG antibody demonstrates that MNS1 immunoprecipitates CCDC114 (A-B) and CCDC114 immunoprecipitates MNS1 (C-D). The open reading frames of recombinant MNS1 and CCDC114 are 495 and 670 amino acids, respectively. The observed approximate molecular weights of 70 and 85 kDa, respectively, represent additional sequence from myc- and FLAG epitope tags. Equal volumes (12 μl) of lysate and immunoprecipitate fractions were loaded on the same gel; lysate fractions represent 0.7% of total lysate (1 ml volume) and immunoprecipitate fractions represent 1/15 lysis volume (33 μl resuspension). Magic Mark protein ladder (M) was used to estimate molecular weight of recombinant MNS1 and CCDC114. (E) Yeast two-hybrid screening of MNS1 and CCDC114 reveals direct protein interaction between MNS1 and CCDC114 (black squares) and dimerization of MNS1 (blue square). Interactions were analyzed by assessment of reporter gene (HIS3 and ADE2) activation via growth on selective media (SD-LWAH) and β-galactosidase colorimetric filter lift assays (LacZ reporter gene). A dimerization of CCDC114 (block 1) and a direct protein interaction between CCDC114 and CCDC151 (blocks 2 and 11) as demonstrated previously by Hjeij et al. 2014, are also here demonstrated.