Fig. 3. Polydopamine coating through K+ assistance in delaminating conditions consisting of strong alkaline pH.
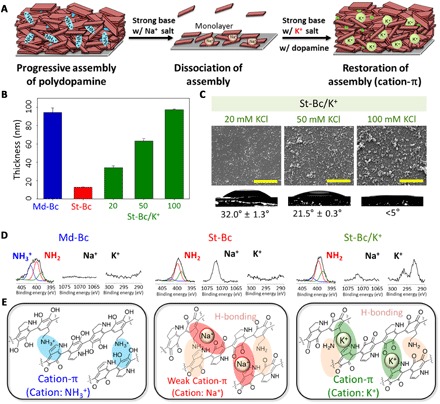
(A) A schematic description for resuming polydopamine coating in the presence of potassium ions after delamination in a strong alkaline condition. (B) The polydopamine coating thickness at pH 8.8 [a method described in (19)] (Md-Bc, blue bar), pH 9.8 (St-Bc, red bar), and pH 9.8 with 20, 50, or 100 mM KCl (St-Bc/K+, green bars). (C) Surface morphology by scanning electron microscopy (SEM) and the static water K+/St-Bc contact angles in increasing KCl concentration [20 mM (left), 50 mM (middle), and 100 mM (right)]. Scale bars, 2 μm. (D) XPS analysis of nitrogen (N1s, –NH3+ in blue, –NH2 in red, and –NH– in green), sodium (Na1s), and potassium (K2p) elements showing cation-to-neutral (–NH3+→–NH2) charge conversion from Md-Bc to St-Bc transfer (that is, elimination of cation-π). The surface-bound Na+ and K+ ions were also detected. (E) Assembly force transitions from strong cation-π interactions between the protonated amines and π system (left, Md-Bc conditions) to weak cation-π interactions between Na+ and the π system due to pH-induced deprotonation (middle, St-Bc conditions). Finally, cation-π interactions are resumed by the addition of K+ under the deprotonation conditions (right, St-Bc/K+ conditions).
