Fig. 4. An application of cation-π–mediated progressive assembly on material-independent superhydrophilic coatings.
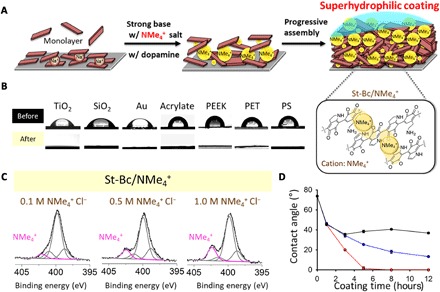
(A) Plausible substructures of polydopamine with ammonium cations (that is, tetramethylammonium, NMe4+) via cation-π interactions between the polydopamine progressive building block indole ring and NMe4+ under St-Bc conditions, resulting in a superhydrophilic coating. (B) The static water contact angle for a variety of substrates without any treatment (top row) and one with a polydopamine/NMe4+ coating on the surface as a superhydrophilic modifier (bottom row). PEEK, polyether ether ketone; PS, polystyrene. (C) The chemical status of nitrogen elements in the polydopamine coating at St-Bc pH in the presence of NMe4+ (concentrations of 0.1, 0.5, or 1.0 M). The amount of the intercalated ammonium (indicated as a signature peak at 402.3 eV) increased with the presence of coatings with higher concentrations of NMe4+. (D) Changes in the water contact angle according to the NMe4+ Cl− concentrations and coating times (red line for 1 M NMe4+, blue line for 0.5 M NMe4+, and black line for 0.1 M NMe4+). A water contact angle below 5° is defined as superhydrophilic.
