Figure 4: End joining in the presence of a synthetic tandem dimer of XLF.
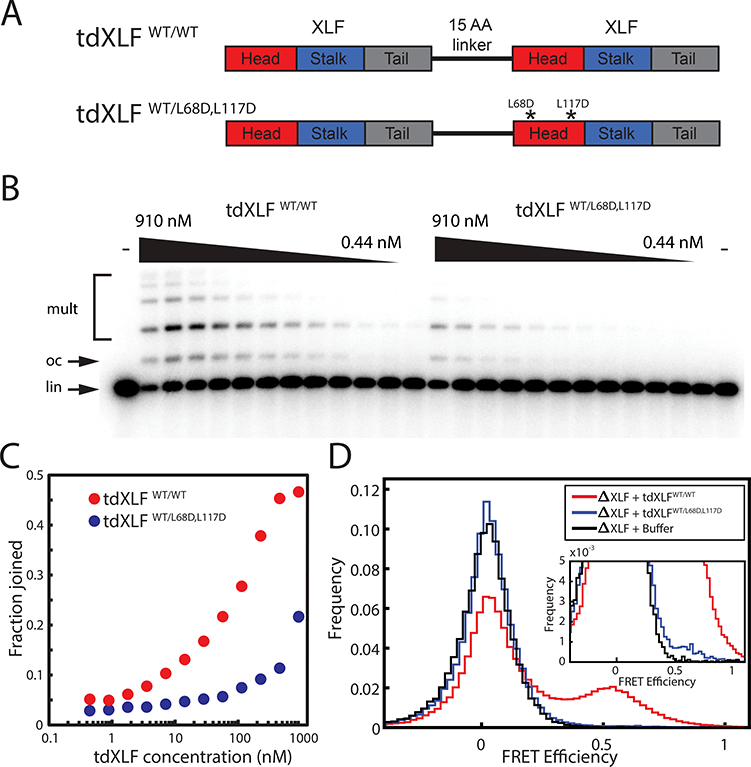
(A) Schematic of XLF tandem dimer (tdXLF) construct. Two XLF coding sequences are concatenated by a flexible 15-amino acid-long linker. Asterisks denote point mutations.
(B) Dose-dependence of end joining as a function of tdXLF concentration for WT/WT and WT/L68D,L117D constructs. Black wedges represent 2-fold serial dilution series from 910 nM to 0.44 nM. Control reactions not supplemented with tdXLF are labeled “-”. lin, linear DNA substrate; oc, open-circular product; mult, dimeric and multimeric products. An uncropped image of B is in Supplementary Data Set 1.
(C) Quantification of product formation in the gel in panel (B). Fraction joined was quantified as background-subtracted intensity of product bands divided by total background-subtracted intensity of substrate and product bands.
(D) Histogram of FRET efficiency from tdXLF rescue of smFRET intramolecular circularization experiments, accumulated over 20 min.
