Figure 1. The basic region can be replaced by a cationic cell-penetrating motif.
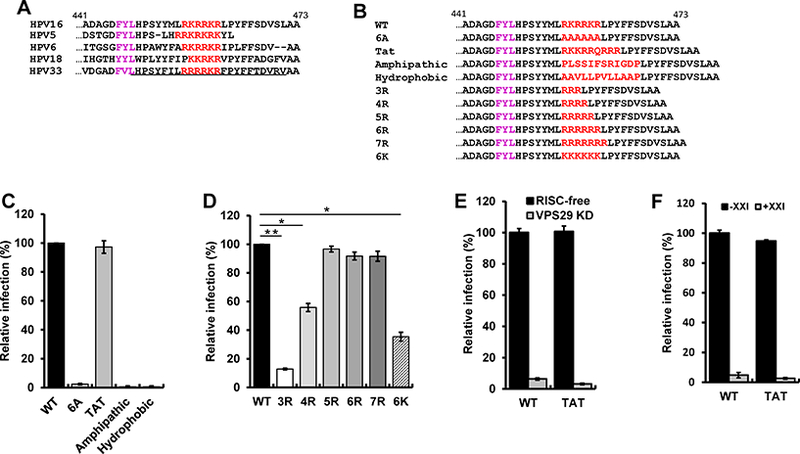
(A) Sequence of the C-terminus of the L2 protein of various HPV types. Basic amino acids (red) downstream of the major FYL retromer binding site (purple) are shown. Numbers indicate position in the HPV16 L2 protein. The membrane-destabilizing sequence in HPV33 L2 is underlined. (B) Sequence of the C-terminus of wild-type and mutant HPV16 L2 proteins. (C and D) HeLa S3 cells were infected with wild-type (WT) or mutant HPV16 PsV stocks containing equal numbers of the HcRed reporter plasmid (corresponding to MOI of one for wild-type). Forty-eight hpi, flow cytometry was used to determine the fraction of fluorescent cells. The results were normalized to the fraction of cells infected by wild-type. The mean results and standard deviation of three or more independent experiments for each sample are shown. *p<0.05; **p<0.01. (E) HeLa S3 cells were transfected with RISC-free control siRNA (black bars) or siRNA targeting Vps29 (grey bars), followed by infection at MOI of one with wild-type or L2-Tat HPV16 PsV. Infectivity was measured (n=3) and displayed as in panel (C). (F) HeLa S3 cells were untreated (black bars) or treated with 250 nM γ-secretase inhibitor XXI for one h at 37°C (grey bars), and then infected as in panel E. Infectivity was measured (n=3) and displayed as in panel C. See also Figure S1 and Table S1.
