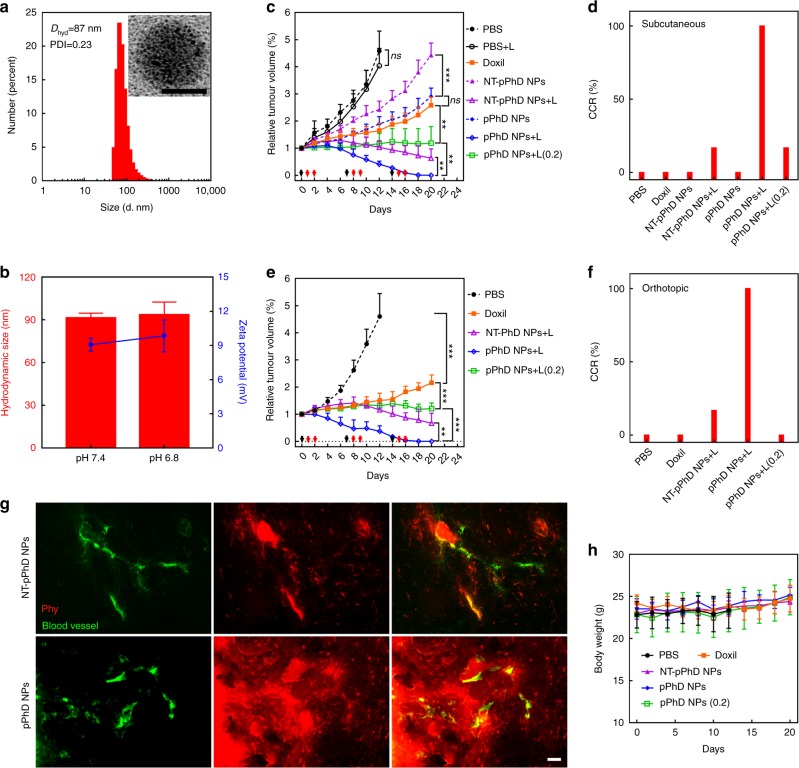
Fig. 6.
In vivo investigation of transformability, chemotherapeutic delivery and photodynamic effect. a Size distribution and morphology of NT-pPhD NPs. The inset is TEM micrograph with a scale bar of 50 nm. b Size and surface charge changes of NT-pPhD NPs before and after the stimulation at pHe (6.8). c The tumour volume changes on subcutaneous tumours (n = 6) after administration of various treatment groups. The black arrows denote the nanoparticles administration, and red ones point out the tumour treated by laser treatments. d The complete cure rate (CCR%) of the subcutaneous tumours. e The tumour volume changes on orthotopic tumours (n = 6). f The CCR% of the orthotopic tumours. g The microscopic distribution of transformable nanoparticles (pPhD NPs) in tumour tissue in comparison with the non-transformable pPhD NPs (NT-pPhD NPs) to prove the superior tumour penetrations. The blood vessel (green) was stained by FITC-dextran (70k) while the red fluorescence indicated the distribution of nanoparticles. The scale bar is 50 µm. h Body weights changes of tumour-bearing mice after treatment (n = 6). The treatment doses were set as 4.7 mg kg−1 for Doxil (calculated by DOX concentration), 5.3 mg kg−1 for Phy, 10 mg/kg for NT-pPhD NPs and 10 mg kg−1 for pPhD NPs (NT-pPhD NPs and pPhD NPs both were calculated based on the concentration of PhD monomer), respectively. For the group of treated with pPhD NPs at low laser dose, the laser (680 nm) dose was set to 0.2 W cm−2 for 6 min. The other laser-treated groups were all set as 0.4 W cm−2 for 3 min. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. All error bars are presented as standard deviation. Note: the mice were immunodeficient, the laser treatments on right tumour were not able to induce immuno-responses to affect the left tumour