Fig. 1.
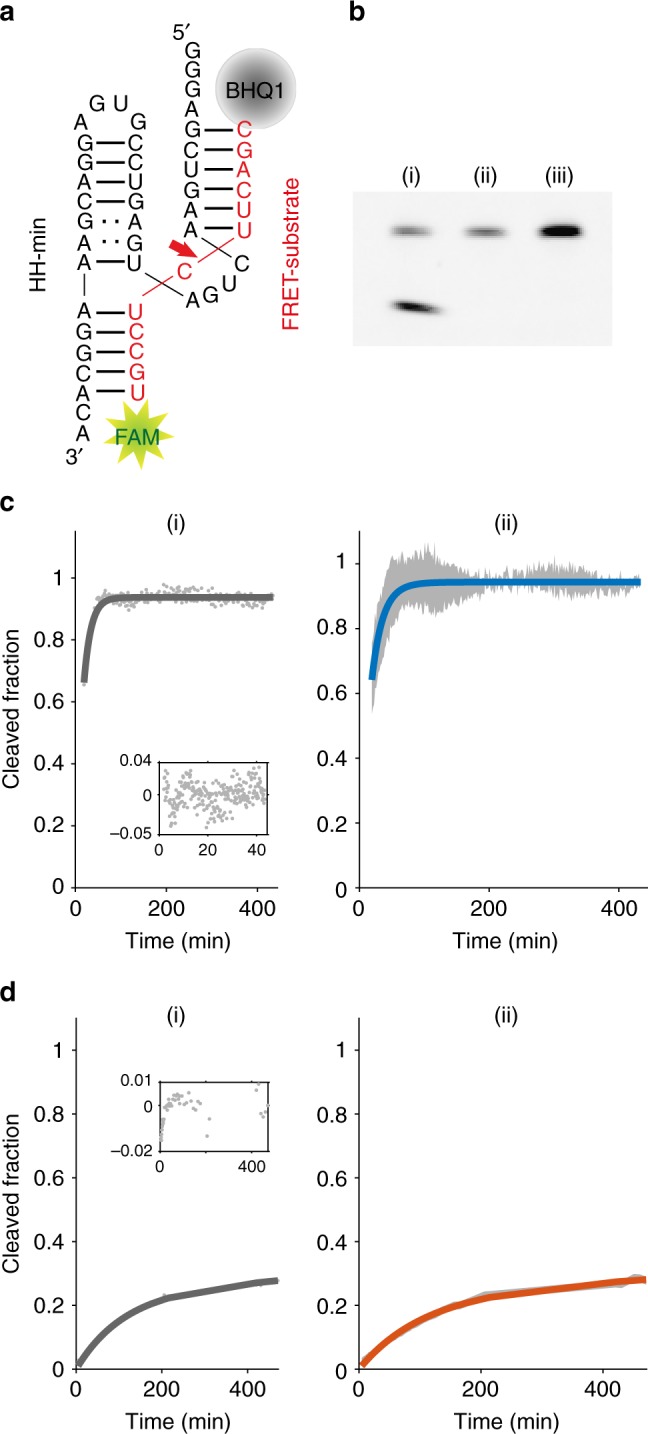
Cleavage of the FRET substrate under different conditions. a HH-min (black) and the FRET substrate (red). b Gel electrophoresis of RNA cleavage in bulk coacervate phase (CM-Dex : PLys, 4:1 final molar ratio); 0.5 μM of FRET substrate was incubated with 1 μM of (i) HH-min, (ii) HH-mut or (iii) no ribozyme in bulk phase (25 °C, 60 min). Samples were analysed by denaturing PAGE followed by fluorescence imaging. The lack of in-gel quenching of the FRET substrate likely results from modifications of BHQ1 during PAGE51. c Real-time cleavage kinetics in 10 mM Tris-HCl pH 8.3 and 4 mM MgCl2. (i) A monoexponential fit (Methods, Eq. 3) (grey line) to kinetic data (grey dots) and residuals of the fit (inset); (ii) mean of the individual fits to each experiment (Blue line) with the standard deviation of the mean of the fits (grey data points) (N = 5). d Cleavage in bulk coacervate phase (normalised to the amount of cleaved product at t = 530 min from gel electrophoresis). (i) Biexponential fit (Methods, Eq. 4) (dark grey line) to experimental data (grey dots) with the residuals (inset); (ii) mean biexponential fit (orange) of individual fits (N ≥ 5). Grey data points represent the standard deviation (N = 5) from the experimental data
