Table 1.
In vitro data for CB1 antagonists – sulfonamides, carbamates & imines
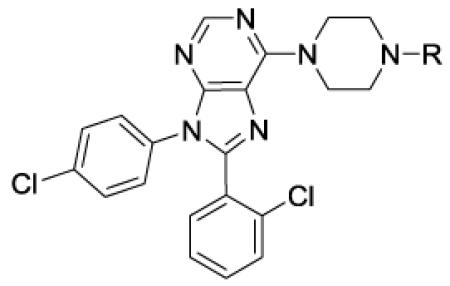
| ||||||
|---|---|---|---|---|---|---|
| # | R | Ke hCB1 (nM) |
Ki hCB1 (nM)a |
Ki hCB2 (nM)a |
Selectivity Ki CB2/CB1 |
MDCK-mdr1 A to B (%)b |
| 2 | Otenabantc | 0.2 | 0.7 | 7700 | 11000 | |
| 5 | Me | 120 | 3d | |||
| 9 | MeSO2 | 3 | 24 | 1700 | 68 | 14 |
| 10 | EtSO2 | 17 | 3 | 980 | 330 | 18 |
| 11 | i-PrSO2 | 1 | 1.4 | 980 | 700 | 11 |
| 12 | c-PrSO2 | 17 | 5 | 2300 | 460 | 4 |
| 13 | n-PrSO2 | 2 | 1.2 | 850 | 710 | 6 |
| 14 | CF3CH2CH2SO2 | 15 | 1.2 | 500 | 420 | |
| 15 | i-BuSO2 | 0.5 | 2 | 2800 | 1400 | 0 |
| 16 | n-BuSO2 | 0.9 | 0.6 | 500 | 830 | 5 |
| 17 | MeOCH2CH2SO2 | 50 | ||||
| 18 | c-HexCH2SO2 | 0.6 | 1.7 | 91 | 54 | |
| 19 |
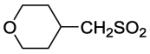
|
4 | 3 | 3000 | 1000 | 9 |
| 20 | MeO2C | 98 | ||||
| 21 | t-BuO2C | 4 | 5 | 3400 | 680 | 3 |
| 22 | MeOCH2CH2O2C | 54 | ||||
| 23 | c-BuO2C | 10 | 3 | 3400 | 1100 | |
| 24 |
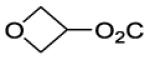
|
95 | ||||
| 25 |
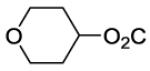
|
105 | ||||
| 26 | c-BuCH2O2C | 12 | 3 | 4600 | 1500 | 0 |
| 27 | 4-F-Ph(HN=)C | 4000 | ||||
| 28 | 4-F-PhNH(HN=)C | 510 | 0.4 | |||
