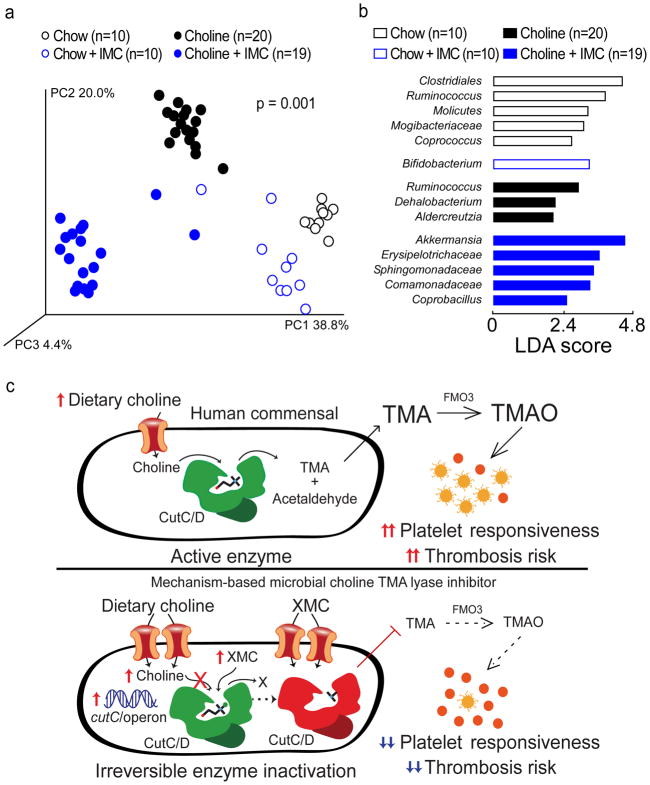
Figure 5. Microbial choline TMA lyase inhibitor reverses diet-induced changes in cecal microbial community composition associated with plasma TMAO levels, platelet responsiveness, and in vivo thrombosis potential.
a–b) Groups of mice were maintained on the indicated diets ± IMC (0.06% w/w) for 2 weeks. Cecal contents were harvested and intestinal microbial community composition was assessed by (a) Principal Coordinates Analysis or (b) Linear Discriminant Analysis (LDA) effect size (LEfSe).
c) (Top) Scheme illustrating the relationship between human gut commensal choline TMA lyase activity, TMA and TMAO generation, and enhanced platelet responsiveness and thrombosis risk in the host. (Bottom) Illustration of the impact of halomethylcholine mechanism-based microbial choline TMA lyase inhibitors (XMC) on human commensal TMA generation, host TMAO generation, platelet responsiveness, and thrombosis potential. Upon irreversible enzymatic inhibition of CutC, microbial cytosolic choline increases. Choline is sensed as an abundant nutrient, leading to upregulation of the cut gene cluster, including cutC and a choline active-transporter. Choline and the halomethylcholine inhibitor are actively pumped into the microbe and accumulate. Sequestration of choline in the microbe also depletes the levels of choline available to neighboring microbes, further preventing production of TMA from the gut microbial community and contributing to a reduction in systemic TMA and TMAO levels in the host. The net effect is reduction in platelet aggregation responsiveness to multiple agonists and reduced thrombosis potential in the host.