Glia are a heterogeneous collection of cell types in the central nervous system (CNS) that continue to surprise in their diversity and functional capability. As intermediaries between vasculature and energy-intensive neurons, and as a result of their presence at the synapse, glia have established a reputation as primary support cells for neurons, providing energy substrates and eliminating excess neurotransmitters. As research tools allow us a more nuanced picture of the CNS, we are beginning to appreciate the specialization, yet broad function of glial cells. Without question, improvements in our understanding of the capabilities of CNS glia will lead to fundamental shifts in neuroscience, not least of which will be to provide another front in the identification of targets for CNS disease intervention.
One such CNS disorder that stands to benefit from glial research is glaucoma. Glaucoma, an age-related neurodegenerative disorder, is the second leading cause of irreversible blindness (Quigley and Broman, 2006). Glaucoma is thought to blind through retinal ganglion cell (RGC) degeneration secondary to axon insult at the optic nerve head (Quigley et al., 1981; Howell et al., 2007); glia are critical players for both.
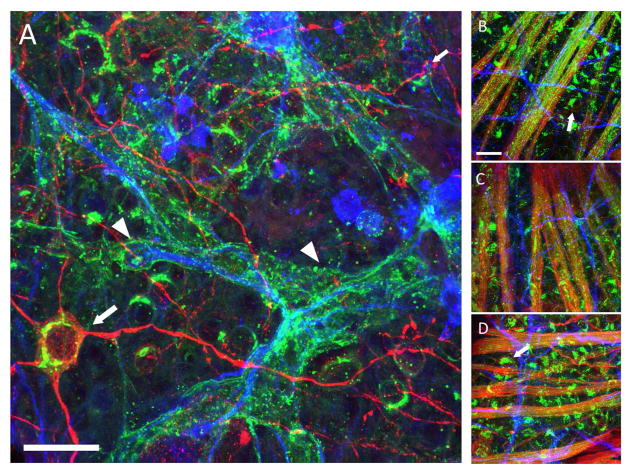
We have evidence to suggest that astrocytes express GM1 gangliosides during retinal ganglion cell degeneration in the context of glaucoma. Retinal ganglion cells (RGCs), the projection neurons in the retina, express GM1, as determined by their efficient and specific uptake of cholera toxin-subunit B (CTB). In the DBA/2J mouse model of glaucoma, astrocytes begin to express GM1 and bind CTB at the same time RGCs show signs of degeneration, including accumulation of phosphorylated neurofilament, (Buckingham et al., 2008; Soto et al., 2008) loss of action potential generation (Baltan et al., 2010) and diminished dendritic arborization (Jakobs et al., 2005). Dysfunctional RGCs, identified by their accumulation of phosphorylated neurofilament (Soto et al., 2008), and lack of retrograde Fluorogold label, also lose CTB binding, suggesting they stop expressing GM1. Prior to degenerative changes in retina, astrocytes do not express GM1, which we determined by their inability to bind CTB (Figure 1). Astrocytic CTB-binding is not age-related, as evidenced by CTB labeling of RGCs and their axons alone in aged DBA/2-Gpnmb+ retina (Figure 1). We are investigating whether gangliosides are upregulated specifically in astrocytes as a result of local neural degeneration because such an occurrence could have profound implications for the survival and function of RGCs.
Figure 1.
Changes in GM1 ganglioside in the DBA2/J and DBA/2-Gpnmb+ retina as determined by cholera toxin-B (CTB) binding. Panels show CTB (green, AlexaFluor-488) labeling in the retina, with β-Tubulin III (red, AlexaFluor-594) and GFAP (blue, AlexaFluor-647) to label retinal ganglion cells (RGCs) and astrocytes, respectively. A. A 13 month-old DBA/2J retina with CTB-positive astrocytes (arrowheads). Arrow points to a β-Tubulin III-positive retinal ganglion cell. B. A 3 month-old DBA/2J retina showing RGC axon fascicles (bundles of green) and no CTB-positive astrocytes. Arrows point to CTB-positive RGCs. C. A 3 month-old DBA/2-Gpnmb+ retina, also with CTB-positive RGC axon fascicles and GFAP-positive astrocytes. D. A 13 month-old DBA/2-Gpnmb+ retina that appears similar to the 3-month-old retina. Arrows point to CTB-positive RGCs. The GFAP-positive astrocytes do not have CTB labeling. The astrocytic CTB labeling occurs only in glaucomatous (panel A), degenerating retina. Scale bar = 20 Jm
Gangliosides are made in the endoplasmic reticulum and further modified in the Golgi by sequential addition of carbohydrate moieties (Yu et al., 2011). Ganglioside expression changes significantly in the course of development, primarily due to the developmental changes in ganglioside synthases (Yu et al., 2004). Adult mammals express greater numbers of gangliosides overall, and those expressed are more complex.
Gangliosides are found in glycolipid-enriched microdomains, or rafts, which can include GPI-linked proteins such as Thy1, glycosphingolipids, caveolin, IgE receptors and other membrane components (Pike, 2009). GM1, when bound to cholera toxin, forms large clusters that redistribute to electron dense areas of the cell membrane that are specialized for signal propagation and clathrin-mediated endocytosis (Wilson et al., 2004). These data suggest increased GM1 expression in astrocytes has implications for cell signaling as well as membrane dynamics.
Lipid raft-bound gangliosides are connected to a wide variety of cellular functions including immune function modulation (Ohmi et al., 2009), growth factor receptor regulation, and maintaining and repairing the CNS (Ohmi et al., 2011). Nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) have been considered as targets for treating glaucoma. In retina, NGF has been shown to change its expression in glaucomic conditions, and to play a neuroprotective role in mice with induced glaucoma (Lambiase et al., 2009; Wang et al., 2014). BDNF haploinsufficiency accelerates degenerative changes in aging mice, such as older DBA/2J (Gupta et al., 2014). BDNF is a neuroprotective agent in glaucomic mice (Domenici et al., 2014), and after optic nerve crush (Di Polo et al., 1998). The ganglioside GM1 has specific interactions with BDNF and NGF that makes it of special interest. GM1 plays a role in antidepressant-like effects in some mouse models of depression, and this change involves the BDNF cascade (Jiang et al., 2016). This identifies a link between BDNF and GM1. We observe a shift in GM1 in the retina as it undergoes glaucomatous degeneration, making it possible that BDNF activity is interrupted as well. The NGF receptor, TrkA, has been shown to associate closely with GM1; NGF binding affinity increases when TrkA and GM1 are linked (Fukuda et al., 2015). The interactions between GM1 and BDNF, or GM1 and NGF suggest that gangliosides are important to our understanding of glaucoma. The loss or interruption of the regulatory actions of GM1 may be a factor in the clinical trial shortcomings for NGF based drugs (Wang et al., 2014). Our characterization work on GM1 in the glaucomic retina will help us further understand this molecule in pathology so we can investigate the interactions among GM1, growth factors and glaucomic degeneration.
The association among GM1 and growth factors has already led to investigations in other neurodegenerative disorders. Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease have provided insights into the pathogenesis of glaucoma, and all have abnormalities associated with the GM1 ganglioside. In Huntington’s disease (HD), fibroblasts taken from patients with HD have lower levels of GM1 than those from healthy individuals (Maglione et al., 2010). When GM1 is given to HD cells in vitro, there is an increase in AKT phosphorylation (Maglione et al., 2010), a result of insulin-like growth factor-1 activation that prevents neuronal death in HD (Humbert et al., 2002). In vivo, GM1 treatment can rescue motor function in a murine model of HD (Di Pardo et al., 2012). In Parkinson’s disease (PD), a deficiency of GM1 has been shown to correlate with the disease (Wu et al., 2012), the basis of which led to a clinical trial using GM1 as a treatment for PD that has shown some positive results (Schneider et al., 2010). The mechanism of the improvement in PD patients from GM1 treatment may involve the association of GM1 with BDNF and NGF, because the substantia nigra has shown decreases in BDNF and NGF in PD (Mogi et al., 1999). Alzheimer’s disease (AD) highlights another possible way that dysregulated GM1 expression might lead to neurodegeneration. In AD, toxic amyloid-β (Aβ) protein fibrillation is catalyzed by binding to GM1 when Aβ is released from damaged neurons (Okada et al., 2007). This has led to investigations into how the association takes place (Fernández-Pérez et al., 2017), and how, once associated, Aβ affects GM1 mobility, potentially making it easier for future binding of more toxic Aβ fibers (Calamai et al., 2016). This is all of particular interest for glaucoma as Aβ has been shown recently to play a role in glaucoma as well as AD (Goldblum et al., 2007; Guo et al., 2007). The roles that GM1 association plays with other neurodegenerative diseases, and its contribution to disease mechanisms there suggests that it could play an important role in glaucomic pathology.
The involvement of GM1 ganglioside in several neurodegenerative diseases indicates many mechanisms to impact cell survival in diseased states. Our observations about the changing distribution of GM1 in glaucoma provide another way to investigate glaucoma, and another tool to potentially treat the disease. The beneficial effects of growth factor signaling on cell survival in other neurodegenerative disorders and the ability of GM1 to affect protein aggregation suggest possible mechanisms for GM1 in modulating glaucomic degeneration.
References
- Baltan S, Inman DM, Danilov C, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci. 2010;30:5644–5652. doi: 10.1523/JNEUROSCI.5956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert WS, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamai M, Evangelisti E, Cascella R, Parenti N, Cecchi C, Stefani M, Pavone F. Single molecule experiments emphasize GM1 as a key player of the different cytotoxicity of structurally distinct Aβ1-42 oligomers. Biochim Biophys Acta - Biomembr. 2016;1858:386–392. doi: 10.1016/j.bbamem.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Di Pardo A, Maglione V, Alpaugh M, Horkey M, Atwal RS, Sassone J, Ciammola A, Steffan JS, Fouad K, Truant R, Sipione S. Ganglioside GM1 induces phosphorylation of mutant huntingtin and restores normal motor behavior in Huntington disease mice. Proc Natl Acad Sci U S A. 2012;109:3528–3533. doi: 10.1073/pnas.1114502109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici L, Origlia N, Falsini B, Cerri E, Barloscio D, Fabiani C, Sans?? M, Giovannini L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS One. 2014;9:1–25. doi: 10.1371/journal.pone.0115579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Pérez EJ, Sepúlveda FJ, Peoples R, Aguayo LG. Role of membrane GM1 on early neuronal membrane actions of Aβ during onset of Alzheimer’s disease. Biochim Biophys Acta - Mol Basis Dis. 2017;1863:3105–3116. doi: 10.1016/j.bbadis.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Fukui T, Hikichi C, Ishikawa T, Murate K, Adachi T, Imai H, Fukuhara K, Ueda A, Kaplan AP, Mutoh T. Neurotropin promotes NGF signaling through interaction of GM1 ganglioside with Trk neurotrophin receptor in PC12 cells. Brain Res. 2015;1596:13–21. doi: 10.1016/j.brainres.2014.11.041. [DOI] [PubMed] [Google Scholar]
- Goldblum D, Kipfer-Kauer A, Sarra GM, Wolf S, Frueh BE. Distribution of amyloid precursor protein and amyloid-β immunoreactivity in DBA/2J glaucomatous mouse retinas. Investig Ophthalmol Vis Sci. 2007;48:5085–5090. doi: 10.1167/iovs.06-1249. [DOI] [PubMed] [Google Scholar]
- Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. doi: 10.1073/pnas.0703707104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, You Y, Li J, Gupta V, Golzan M, Klistorner A, van den Buuse M, Graham S. BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochim Biophys Acta. 2014;1842:1567–1578. doi: 10.1016/j.bbadis.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert S, Bryson EA, Cordeliè Res FP, Connors NC, Datta SR, Finkbeiner S, Greenberg ME, Dé F, Saudou R. The IGF-1/Akt Pathway Is Neuroprotective in Huntington’s Disease and Involves Huntingtin Phosphorylation by Akt. Dev Cell. 2002;2:831–837. doi: 10.1016/s1534-5807(02)00188-0. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Song L, Wang CN, Zhang W, Huang C, Tong LJ. Antidepressant-like effects of GM1 ganglioside involving the BDNF signaling cascade in mice. Int J Neuropsychopharmacol. 2016;19:1–13. doi: 10.1093/ijnp/pyw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Aloe L, Centofanti M, Parisi V, Mantelli F, Colafrancesco V, Manni G, Bucci M, Bonini S, Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci USA. 2009;106:13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione V, Marchi P, Di Pardo A, Lingrell S, Horkey M, Tidmarsh E, Sipione S. Impaired Ganglioside Metabolism in Huntington’s Disease and Neuroprotective Role of GM1. J Neurosci. 2010;30:4072–4080. doi: 10.1523/JNEUROSCI.6348-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- Ohmi Y, Tajima O, Ohkawa Y, Mori A, Sugiura Y, Furukawa K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance. PNAS. 2009;106:1–6. doi: 10.1073/pnas.0912336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi Y, Tajima O, Ohkawa Y, Yamauchi Y, Sugiura Y, Furukawa K, Furukawa K. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice. J Neurochem. 2011;116:926–935. doi: 10.1111/j.1471-4159.2010.07067.x. [DOI] [PubMed] [Google Scholar]
- Okada T, Wakabayashi M, Ikeda K, Matsuzaki K. Formation of Toxic Fibrils of Alzheimer’s Amyloid β-Protein-(1-40) by Monosialoganglioside GM1, a Neuronal Membrane Component. J Mol Biol. 2007;371:481–489. doi: 10.1016/j.jmb.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–8. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Sendek S, Daskalakis C, Cambi F. GM1 ganglioside in Parkinson’s disease: Results of a five year open study. J Neurol Sci. 2010;292:45–51. doi: 10.1016/j.jns.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Soto I, Oglesby E, Buckingham BP, Son JL, Roberson EDO, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008:28. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang R, Thrimawithana T, Little PJ, Xu J, Feng Z-P, Zheng W. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int. 2014;2014:759473. doi: 10.1155/2014/759473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, Samelson LE, Yang L, Kotula PG, Oliver JM. Markers for Detergent-resistant Lipid Rafts Occupy Distinct and Dynamic Domains in Native Membranes. Mol Biol Cell. 2004;15:2580–2592. doi: 10.1091/mbc.E03-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lu Z-H, Kulkarni N, Ledeen RW. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J Neurosci Res. 2012;90:1997–2008. doi: 10.1002/jnr.23090. [DOI] [PubMed] [Google Scholar]
- Yu RK, Bieberich E, Xia T, Zeng G. Regulation of ganglioside biosynthesis in the nervous system. J Lipid Res. 2004;45:783–794. doi: 10.1194/jlr.R300020-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu RK, Tsai Y-T, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides-an overview. J Oleo Sci. 2011;60:537–544. doi: 10.5650/jos.60.537. [DOI] [PMC free article] [PubMed] [Google Scholar]