Figure 1.
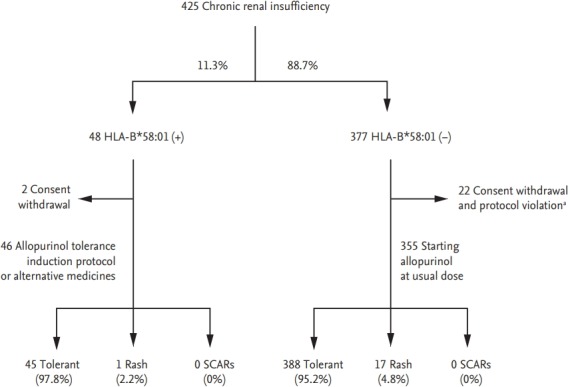
Allopurinol was administered according to a tolerance induction protocol or substituted for an alternative medication in 46 patients with the HLA-B*58:01 allele. During the 90-day period of drug administration, none of 46 patients with the HLA-B*58:01 allele developed severe cutaneous adverse reaction (SCARs). Adapted from Jung et al., with permission from Springer Nature [50]. HLA, human leukocyte antigen. a Withdrawal of allopurinol regardless of hypersensitivity.
