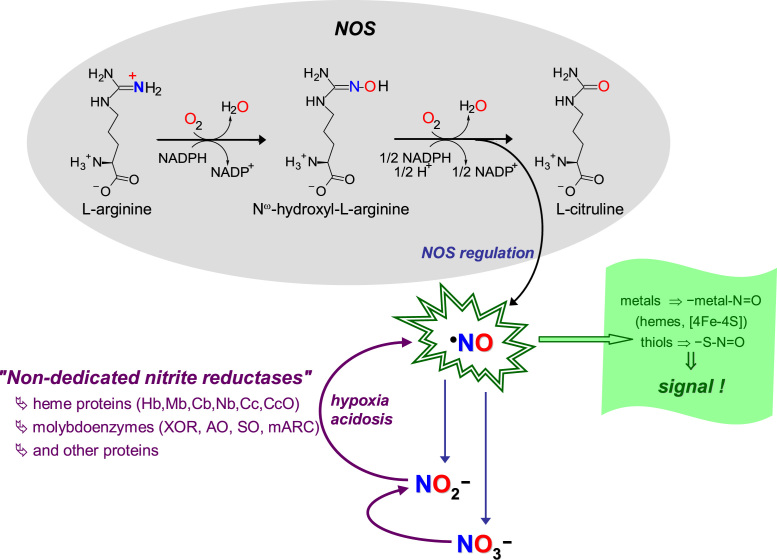
Fig. 2.
"Classic" and novel pathways of NO formation. The "classic" pathways of NO formation (black arrows, grey shadowed area) are catalyzed by NOS, complex homodimeric enzymes, constituted by one flavinic reductase C-terminal domain and one hemic oxygenase N-terminal domain. During catalysis, NO is formed from one oxygen atom of dioxygen (printed in red) and from the guanidinium nitrogen atom of L-arginine (in blue), and three reducing equivalents are consumed (in the form of NADPH). The electrons from NADPH are transferred through the reductase domain to the b heme iron of the oxygenase domain; on the heme, the dioxygen is activated to hydroxylate L-arginine; the Nω-hydroxy-L-arginine formed is, then, oxidized to yield L-citrulline and NO. To control the specificity of NO signaling (indigo arrows and text), and also to limit the NO toxicity, NOS are tightly regulated and the NO life time is controlled through its rapid oxidation to nitrate and nitrite. The novel pathways of NO formation (violet arrows and text) are reductive in nature (contrary to the oxidative NOS-catalyzed pathways) and are dependent on the nitrite reduction under hypoxic and anoxic conditions. These pathways are catalyzed by "non-dedicated nitrite reductases", metalloproteins that are present in cells to perform other functions, including several heme proteins and molybdoenzymes. The NO biological effects are accomplished (green arrows and text), mainly, by post-translational modification of cysteine residues and other thiols and of transition metal centers, mostly labile [4Fe-4S] centers and hemes (as is the case of the well known activation of guanylate cyclase), to yield nitrosothiol (-S-N=O) and nitrosyl (-metal-N=O) derivates, respectively.