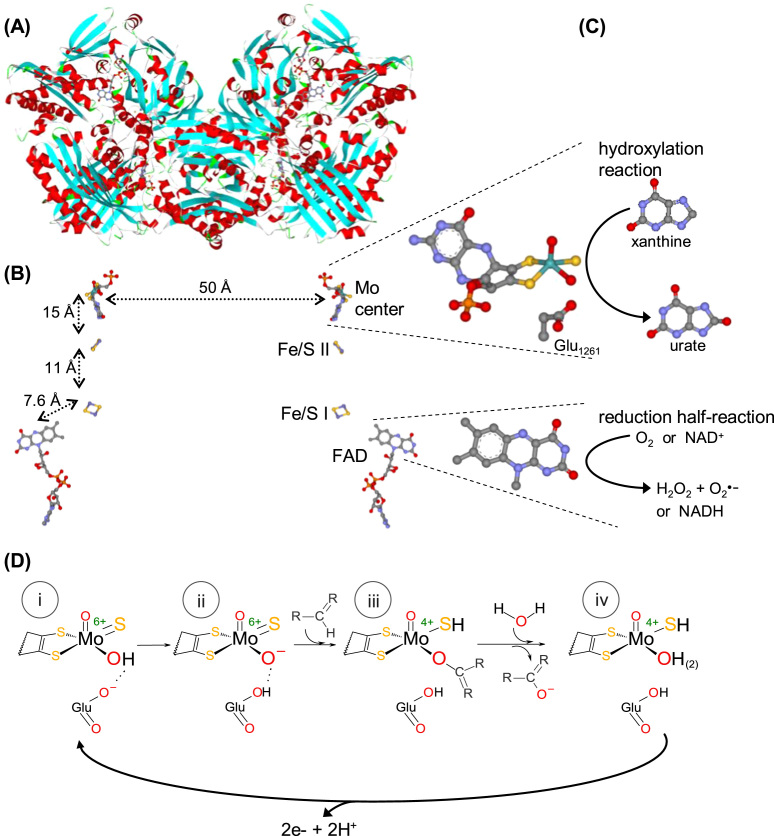
Fig. 3.
XOR structure and reactivity. (A) Three-dimensional structure view of the bovine milk XO homodimer. (B) Arrangement of the four redox-active centers shown in the same orientation as in (A). The four centers are identified on the monomer on the right, and the distances between adjacent centers are shown on the monomer on the left. The molybdenum center, together with the conserved glutamate residue, and the FAD isoalloxazine ring are zoomed on the right. (C) The oxidation and reduction half-reactions are represented next to the centers where they are catalyzed. (D) The molecular reaction mechanism of XOR and AO-catalyzed hydroxylation reactions is presently well established [97], [98], [99], [100], [101], [102] and is here exemplified with a R2-C-H molecule that is hydroxylated to R2-C-OH to represent both heterocyclic compounds and linear aldehydes. The hydroxylation catalysis is initiated with the activation of the molybdenum hydroxyl ligand (Mo6+-OH) by a neighboring conserved deprotonated glutamate residue, to form an oxidized Mo6+-O-(=S) core (base-assisted catalysis) (i→ii). It follows the nucleophilic attack of Mo6+-O- on the carbon atom to be hydroxylated, with the simultaneous hydride transfer from substrate to the sulfo ligand (Mo6+=S → Mo4+-SH), resulting in the formation of a covalent intermediate, Mo4+-O-C-R2(-SH) (ii→iii). The subsequent hydrolysis of the Mo4+-O bond releases the product hydroxylated and yields a Mo4+-OH(2)(-SH) core (oxidation half-reaction) (iii→iv). Finally, the two electrons transferred from the substrate to the molybdenum are rapidly transferred, via the Fe/S centers, to the FAD, where the dioxygen or NAD+ reduction takes place (reduction half-reaction) (iv→i). In the now oxidized molybdenum center, the sulfo group is deprotonated and the initial Mo6+-OH(=S) core is regenerated and the catalytic cycle can be reinitiated (i). The structures shown ((A),(B)) are based on the PDB file 1FO4; in (A), α helices and β sheets are shown in red and turquoise, respectively; in (B) and (C), atoms are color coded as follows: carbon, grey; nitrogen, blue; oxygen, red; sulfur, gold; phosphorous, orange; iron, dark gold; molybdenum, cyan.