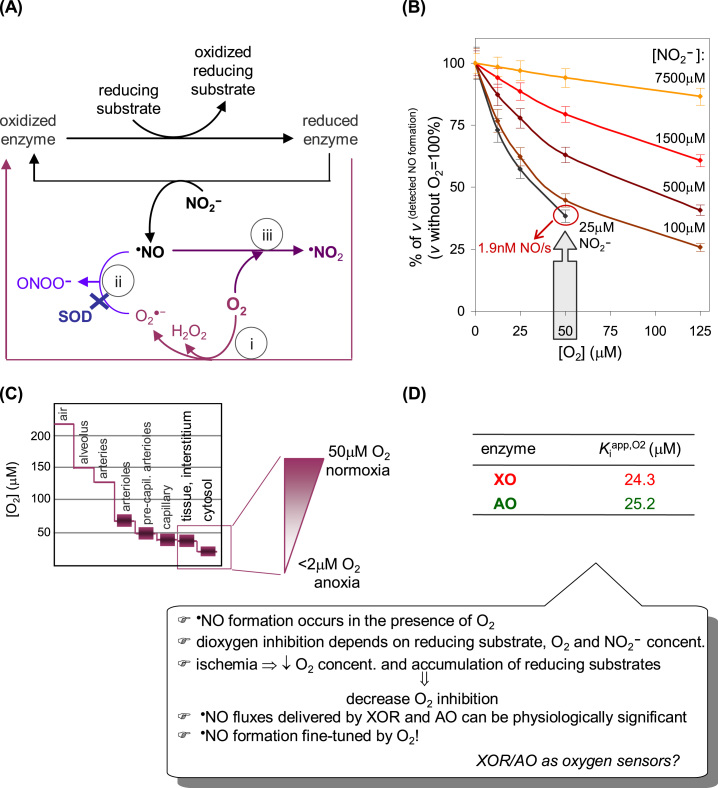
Fig. 8.
Effects of dioxygen on the XOR and AO-dependent NO status. (A) Dioxygen interferes with NO at different levels. (i) Dioxygen is efficiently reduced at the enzymes FAD center (Fig. 3) and rapidly consumes the electrons derived from the reducing substrates. Consequently, dioxygen readily decreases the concentration of enzyme molecules with reduced molybdenum available to react with nitrite, thus decreasing (inhibiting) the NO formation. Theoretically, it can be demonstrated that the dioxygen competitive inhibition constant (Kiapp,O2) is formally equal to the Kmapp,O2 of the hydroxylation reaction. (ii) At the same time, the superoxide anion radical formed (Eqs. (2), (4)) reacts with NO to yield peroxynitrite, in a diffusion controlled reaction (k ≈ 109–1010 M−1 s−1). This effect can be counteracted by the presence of superoxide dismutase (k ≈ 2 × 109 M−1 s0.1), although, in this case, the dioxygen is partially restored in the system. (iii) In addition, dioxygen can also react directly with NO, but in a comparatively slow reaction (k ≈ 106–107 M−2 s−1), to yield different products, including •NO2. This dioxygen effect can not be experimentally counteracted. (B) Data from XO-catalyzed NO formation at pH 6.3; adapted with permission from Ref. [137]. Copyright 2015 American Chemical Society. (C) Scheme modified from Ref. [180]. (D) Data pH 6.3; adapted with permission from Ref. [137]. Copyright 2015 American Chemical Society.