Abstract
Following the evaluation of surgical lung volume reduction (LVR) in the National Emphysema Treatment (NETT) trial, different endoscopic LVR procedures have been developed for severe emphysema. Among those, endobronchial valve placement is the best evaluated method. All these therapies aim at reducing hyperinflation and at improving respiratory mechanics. It has been shown that these procedures can improve quality of life, lung function and exercise capacity in a significant and clinically meaningful way in suitable patients. Optimal medical therapy, physical rehabilitation, smoking cessation and respiratory insufficiency assessments should been thoroughly evaluated by a multi-disciplinary team before considering any LVR procedure. Clinical experience is necessary to decide if a patient is an appropriate candidate for a surgical or an interventional LVR procedure, to choose the optimal treatment strategy and to provide a high-level of care after the intervention, particularly when complications such as pneumothorax or persistent air leak occur in this already severely ill patient population. High volume emphysema care centers, providing a broad spectrum of different LVR procedures and involving a multidisciplinary team in the diagnostic process, are best suited to provide an optimal outcome. The aim of this manuscript is to describe the structures and procedures required to achieve the best possible outcome even in patients with advanced stage of their emphysema disease, including patients who are candidates for lung transplantation.
Keywords: Emphysema, lung volume reduction (LVR), valves, lung volume reduction surgery (LVRS)
Introduction
Chronic obstructive pulmonary disease (COPD) is predicted to become the third most frequent cause of death in the world by 2020 (1). Pulmonary emphysema is an important clinical phenotype of advanced COPD characterized by tissue destruction, hyperinflation and ventilation-perfusion mismatch. With the intention to decrease hyperinflation, to improve ventilatory mechanics and diaphragmatic function, and thus to reduce the work of breathing, surgical and more recently bronchoscopic lung volume reduction (BLVR) procedures have been developed and successfully evaluated for patients with pulmonary emphysema.
The National Emphysema Treatment (NETT) trial has shown that lung volume reduction surgery (LVRS) can reduce mortality and increase exercise capacity in selected patients with heterogenous upper-zone emphysema and reduced exercise tolerance. A moderate increase in exercise capacity was also found in patients with homogenous emphysema and low exercise capacity (2). BLVR procedures (valves, coils, vapor and foam) have been subsequently developed and shown in clinical studies to improve significantly lung function, exercise capacity and quality of life (3-6). For Coils, Vapor and Foam, more evidence is required regarding their optimal treatment selection algorithms.
For optimal outcomes, accurate patient selection, management and postoperative care are critical (7,8), and less well understood in patients with very severe emphysema, particularly because they have been excluded from treatment in many former studies.
An important issue requiring special consideration is the application of lung volume reduction techniques as a bridge or alternative to lung transplantation. Our center is a tertiary referral center for end-stage emphysema patients that offers all treatment options including a lung transplantation program. This manuscript focuses on the structures and procedures required to safely perform bronchoscopic and surgical LVR, with special focus on patients in very advanced stages of their emphysema.
Patient selection
Lung function
A broad experience in bronchoscopic or surgical LVR and optimal selection of patients is crucial for favorable patient outcome. In many clinical studies, patients with an airway obstruction FEV1 >40% and a hyperinflation with RV <200% were included for bronchoscopic or surgical therapy assessments (2-5,9-12). However, in our opinion this does not reflect current selection algorithms, as tissue destruction and hyperinflation have to be severe enough to allow for significant improvements. Regarding coils, a recently published post-hoc analysis of a multi-center trial showed that patients improve after bilateral coil placement only if their baseline RV exceeds 225% predicted (5), which is higher than the value of 150% described for surgical LVR and Vapor ablation (13,14). It is important to emphasize that all LVR modalities are thought to work primarily by reducing lung hyperinflation, or in operational terms, by reducing residual volume. In our and many other emphysema care centers, patients are usually considered for any type of LVR procedure only if they present with values of FEV1 <40% predicted and RV >200% predicted. These limits are supported by the average baseline characteristics in the major trials (7). Additionally, the RV/TLC ratio may provide further guidance if larger, ideally, than 0.58 as described by Caviezel et al. (13). Whether there is a clear lower limit of FEV1 to exclude patients from LVR procedures is still under debate and will be discussed later. A diffusion capacity (DLCO) <20% has been described as a predictor for increased mortality after LVRS and is usually considered a contraindication for surgical LVR (2).
6-minute walk test (6-MWT)
It is important to evaluate exercise capacity for LVR to exclude patients who will not improve after the procedure. The 6-MWT can be performed in a standardized manner and has become established as the method to evaluate exercise capacity (15). Patients with a 6-MWT above 450 m are not physically limited enough to notice a clinical improvement after LVR and should be excluded from any LVR procedure. Patients with a 6-MWT <200 m are pathophysiologically limited most often by reasons other than dynamic hyperinflation, e.g., reduced skeletal muscle strength, obesity or cardiac issues. Those patients should be sent for intense pulmonary rehabilitation before being re-evaluated for LVR (8). Patients who are not limited by dynamic hyperinflation have a low probability of clinical improvement after LVR. Although it has not been proven by prospective trials and is only based on our clinical experience, if the oxygen saturation does not decrease during a 6-MWT, the patient’s exercise capacity may be limited by comorbidities and not by the emphysema. In such a situation LVR should be carefully reconsidered.
Collateral ventilation (patient selection for valves only)
One of the most important results of the VENT trial is that patients with collateral ventilation have to be excluded from BLVR with valves (9). Appropriate patients with no collateral ventilation can expect significant and clinically meaningful improvements in lung function, exercise capacity and quality of life from BLVR with valves (4). When a bronchoscopic procedure with valves is considered, the collateral ventilation status has to be evaluated before making a decision on the appropriate LVR procedure (7). Measuring collateral ventilation by a bronchoscopic procedure (CHARTIS©) predicts successful LVR in 70–90% of patients (4,16,17). A surrogate for the presence of collateral ventilation is an incomplete fissure between two lobes assessed on CT scan. While visual analysis of the fissures may be erroneous, software-assisted evaluation together with quantitative CT scan (QCT) can quantify the completeness of the fissure. Fissure completeness below 80% predicts the presence of collateral ventilation and a low probability of successful bronchoscopic LVR with valves. In this situation CHARTIS measurement might not be necessary (18). These patients should be considered for surgical LVR or alternative bronchoscopic methods.
CT scan
The HR-CT scan should be assessed not only for collateral ventilation but also for severity of lobar emphysema. Other pulmonary findings such as significant bronchiectasis and fibrosis disqualify patients for any LVR procedure. A suspicious nodule in the target lobe favors a surgical approach if the patient otherwise qualifies for LVRS. If the CT scan reveals the emphysema is mainly caused by secondary LVR of an adjacent area (i.e., bronchiectasis), patients should not be subjected to further LVR evaluation.
Smoking cessation
Patients who are continuous smokers should be excluded from any LVR procedures. This is more an ethical than evidence-based question. As continuous smoking doubles the loss of lung function over time compared to patients who stopped smoking, any post-treatment lung function improvement gained will vanish within a few years (19). The decision to offer a LVR procedure is a good time to motivate COPD patients to quit smoking.
Multidisciplinary board (MDB)
In cancer treatment, MDB s are routinely established and lead to improved treatment decisions compared to decisions made by the single specialist. In emphysema treatment, where interventions are performed purely for functional reasons, MDB have been established only in a few dedicated centers (20).
For the treatment of emphysema patients, endoscopic treatment is frequently performed by interventional pulmonologists whereas surgical LVR is performed by thoracic surgeons. A MDB should at least be attended by thoracic surgeons, interventional pulmonologists and radiologists, to cover all involved specialties, and to guide optimal treatment decisions. Ideally, all patients should be discussed in a MDB prior to any LVR intervention (Figure 1). In our center, a MDB that meets on a weekly basis was established in 2016.
Figure 1.
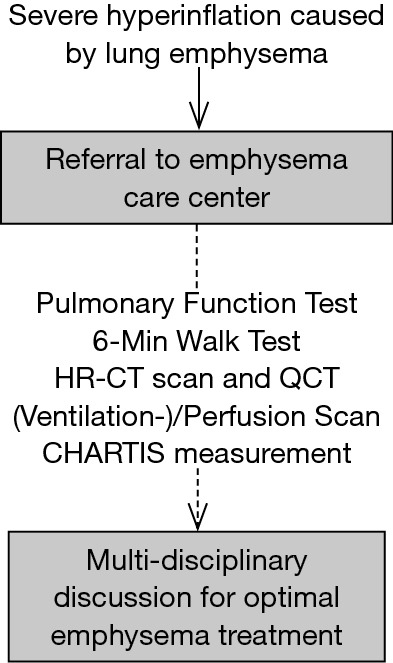
Patient selection and assessment should be standardized and transparent. Decisions should be made by a multi-disciplinary team discussion that includes all specialists responsible for emphysema treatment.
Patient management
Patients with severe emphysema suffer from significantly impaired gas exchange and diminished ventilatory capacity. Comorbidities are also frequent in these patients, resulting in an increased risk of peri-interventional complications from any form of LVR procedure. Unstable patients with active infection or cardiac insufficiency should be stabilized before the intervention.
Medical therapy
Pharmacological interventions with bronchodilators significantly improve lung function and hyperinflation at rest and during exercise and reduce the intensity of dyspnea in patients with COPD (21,22). Although Stage IV COPD patients have been assessed only in few randomized controlled trials, these patients should have been under optimal pharmacological treatment according to the GOLD guidelines (1). Patients should only be considered for LVR therapy after optimal medical therapy does not lead to sufficient improvement of disease severity.
Rehabilitation
Severe COPD is not only linked to lung tissue destruction and respiratory muscle dysfunction, but also to limb muscle dysfunction caused by muscle disuse atrophy resulting from low physical activity, chronic systemic inflammation, nutritional factors, drugs and comorbidities (21,23). Exercise training can significantly increase muscle mass and can have a positive effect on exercise capacity, quality of life and even lung function (24). Patients considered for endoscopic or surgical LVR should be evaluated for limb muscle dysfunction and should undergo a specialized pulmonary rehabilitation program that includes and focuses on a specific exercise-training program. This is especially mandatory when patients are limited not only by dynamic hyperinflation but also because of deconditioning.
Hypoxemia & hypercapnia
Patients with severe hypoxemia or hypercapnia do not have to be excluded from LVR procedures if a significant improvement in blood gas analysis can be achieved, either with supplementary oxygen or non-invasive ventilation. Patients with severe hypoxemia (<55 mmHg, room air) should be treated with supplementary oxygen before performing the LVR procedure. The oxygen flow should be adjusted after the procedure. For patients with significant hypercapnia (>55 mmHg), non-invasive ventilation should be initiated before and adjusted after the procedure. Bronchoscopic LVR can improve respiratory mechanics and increase alveolar ventilation (25).
LVR and lung transplantation
Severe COPD is the most common indication worldwide for lung transplantation. However, when organs are allocated according to the lung allocation score (LAS), as used in Germany and in the USA, patients with COPD have a lower chance of receiving an organ (26). Only limited data are available for endoscopic LVR in patients on waiting lists for lung transplantations. These “Low-FEV1”-patients with an FEV1 ≤20% predicted are severely impaired by lung emphysema with seriously diminished quality of life and have the highest need for therapy (27). Two separate studies evaluating BLVR using endobronchial valves in LOW-FEV1 patients have shown that EBV therapy is feasible and safe, and improvements of lung function and exercise capacity are comparable to patients with less severely diminished lung function (28,29). BLVR with endobronchial valves improves lung function and quality of life of patients awaiting lung transplantation and does not influence the outcome after lung transplantation. BLVR with valves can therefore be considered an adequate bridging strategy to lung transplantation (30,31).
A pneumothorax after BLVR can occur and lead to a critical situation. Immediate attention and chest tube placement can be necessary. This is even more important in patients with Low-FEV1, and close supervision, preferably in the ICU, for at least 24 h after the bronchoscopic procedure is recommended (28). For more than 20 years, surgical LVR has been used as an alternative to lung transplantation, and as a bridging procedure to postpone the need for lung transplantation, as well as an option to improve the patient´s condition prior to transplantation (32-34). While early experience of uniformly good results has been described for lung transplantation after LVRS, two reports from the US raised concerns that the long term outcome might be impaired (35,36). Such impairment, however, does not match the authors’ personal experience and has not been confirmed by others. Further, a recent paper from the Zurich group reports that long term outcome after lung transplantation is not negatively effected by prior LVRS (37). Ultimately, patient selection for both procedures remains a crucial issue in achieving good outcomes.
Conclusions
To achieve the best possible outcome, selection and treatment of patients with severe emphysema should be performed in a setting where all treatment options are available and the involved specialists cooperate in in the framework of an emphysema care center. After careful evaluation and pre-interventional preparation, the most appropriate treatment procedure has to be selected by a MDB. Since further evidence on treatment selection algorithms is needed such a setting facilitates prospective clinical trials. When LVR procedures are performed by experienced teams together with close postoperative patient monitoring, LVR treatments are an efficient therapy that might be offered also to patients with very severe forms of the disease.
Acknowledgements
None.
Footnotes
Conflicts of Interest: K Darwiche received research and travelling grants and speakers fees from PulmonX, PneumRx, Broncus and Nuveira. C Aigner has no conflicts of interest to declare.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. 10.1056/NEJMoa030287 [DOI] [PubMed] [Google Scholar]
- 3.Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185-93. 10.1016/S2213-2600(16)00045-X [DOI] [PubMed] [Google Scholar]
- 4.Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]
- 5.Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA 2016;315:2178-89. 10.1001/jama.2016.6261 [DOI] [PubMed] [Google Scholar]
- 6.Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651-62. 10.1183/09031936.00205614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herth FJF, Slebos DJ, Criner GJ, et al. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation - Update 2017. Respiration 2017;94:380-8. 10.1159/000479379 [DOI] [PubMed] [Google Scholar]
- 8.Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial Valves for Endoscopic Lung Volume Reduction: Best Practice Recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2017;93:138-50. 10.1159/000453588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. 10.1056/NEJMoa0900928 [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. 10.1183/09031936.00161611 [DOI] [PubMed] [Google Scholar]
- 11.Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. 10.1164/rccm.201707-1327OC [DOI] [PubMed] [Google Scholar]
- 12.Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. 10.1164/rccm.201607-1383OC [DOI] [PubMed] [Google Scholar]
- 13.Caviezel C, Franzen D, Inci I, et al. [Lung Volume Reduction Surgery - State of the Art 2016]. Zentralbl Chir 2016;141 Suppl 1:S26-34. 10.1055/s-0042-112026 [DOI] [PubMed] [Google Scholar]
- 14.Valipour A, Herth FJ, Eberhardt R, et al. Design of the randomized, controlled sequential staged treatment of emphysema with upper lobe predominance (STEP-UP) study. BMC Pulm Med 2014;14:190. 10.1186/1471-2466-14-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-7. Erratum in: Am J Respir Crit Care Med 2016;193:1185. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 16.Gompelmann D, Eberhardt R, Michaud G, et al. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010;80:419-25. 10.1159/000319441 [DOI] [PubMed] [Google Scholar]
- 17.Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J 2013;41:302-8. 10.1183/09031936.00015312 [DOI] [PubMed] [Google Scholar]
- 18.Schuhmann M, Raffy P, Yin Y, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med 2015;191:767-74. 10.1164/rccm.201407-1205OC [DOI] [PubMed] [Google Scholar]
- 19.Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med 2000;161:381-90. 10.1164/ajrccm.161.2.9901044 [DOI] [PubMed] [Google Scholar]
- 20.Rathinam S, Oey I, Steiner M, et al. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programme†. Eur J Cardiothorac Surg 2014;46:1021-6; discussion 1026. 10.1093/ejcts/ezu129 [DOI] [PubMed] [Google Scholar]
- 21.Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;189:e15-62. 10.1164/rccm.201402-0373ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:187-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gea J, Pascual S, Casadevall C, et al. Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. J Thorac Dis 2015;7:E418-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greulich T, Koczulla AR, Nell C, et al. Effect of a Three-Week Inpatient Rehabilitation Program on 544 Consecutive Patients with Very Severe COPD: A Retrospective Analysis. Respiration 2015;90:287-92. 10.1159/000436979 [DOI] [PubMed] [Google Scholar]
- 25.Tsujino K, Sasada S, Kodama M, et al. Severe bullous emphysema and hypercapnia successfully treated by bronchoscopic lung volume reduction. Respirology 2009;14:907-9. 10.1111/j.1440-1843.2009.01581.x [DOI] [PubMed] [Google Scholar]
- 26.Lane CR, Tonelli AR. Lung transplantation in chronic obstructive pulmonary disease: patient selection and special considerations. Int J Chron Obstruct Pulmon Dis 2015;10:2137-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000;55:1000-6. 10.1136/thorax.55.12.1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darwiche K, Karpf-Wissel R, Eisenmann S, et al. Bronchoscopic Lung Volume Reduction with Endobronchial Valves in Low-FEV1 Patients. Respiration 2016;92:414-419. 10.1159/000452629 [DOI] [PubMed] [Google Scholar]
- 29.Trudzinski FC, Höink AJ, Leppert D, et al. Endoscopic Lung Volume Reduction Using Endobronchial Valves in Patients with Severe Emphysema and Very Low FEV1. Respiration 2016;92:258-65. 10.1159/000448761 [DOI] [PubMed] [Google Scholar]
- 30.Fuehner T, Clajus C, Fuge J, et al. Lung transplantation after endoscopic lung volume reduction. Respiration 2015;90:243-50. 10.1159/000434685 [DOI] [PubMed] [Google Scholar]
- 31.Venuta F, Diso D, Anile M, et al. Bronchoscopic lung volume reduction as a bridge to lung transplantation in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2011;39:364-7. 10.1016/j.ejcts.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 32.Senbaklavaci O, Wisser W, Ozpeker C, et al. Successful lung volume reduction surgery brings patients into better condition for later lung transplantation. Eur J Cardiothorac Surg 2002;22:363-7. 10.1016/S1010-7940(02)00354-8 [DOI] [PubMed] [Google Scholar]
- 33.Tutic M, Lardinois D, Imfeld S, et al. Lung-volume reduction surgery as an alternative or bridging procedure to lung transplantation. Ann Thorac Surg 2006;82:208-13; discussion 213. 10.1016/j.athoracsur.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 34.Nathan SD, Edwards LB, Barnett SD, et al. Outcomes of COPD lung transplant recipients after lung volume reduction surgery. Chest 2004;126:1569-74. 10.1378/chest.126.5.1569 [DOI] [PubMed] [Google Scholar]
- 35.Shigemura N, Gilbert S, Bhama JK, et al. Lung transplantation after lung volume reduction surgery. Transplantation 2013;96:421-5. 10.1097/TP.0b013e31829853ac [DOI] [PubMed] [Google Scholar]
- 36.Backhus L, Sargent J, Cheng A, et al. Outcomes in lung transplantation after previous lung volume reduction surgery in a contemporary cohort. J Thorac Cardiovasc Surg 2014;147:1678-83.e1. 10.1016/j.jtcvs.2014.01.045 [DOI] [PubMed] [Google Scholar]
- 37.Inci I, Iskender I, Ehrsam J, et al. Previous lung volume reduction surgery does not negatively affect survival after lung transplantation. Eur J Cardiothorac Surg 2018;53:596-602. 10.1093/ejcts/ezx318 [DOI] [PubMed] [Google Scholar]


