Figure 2.
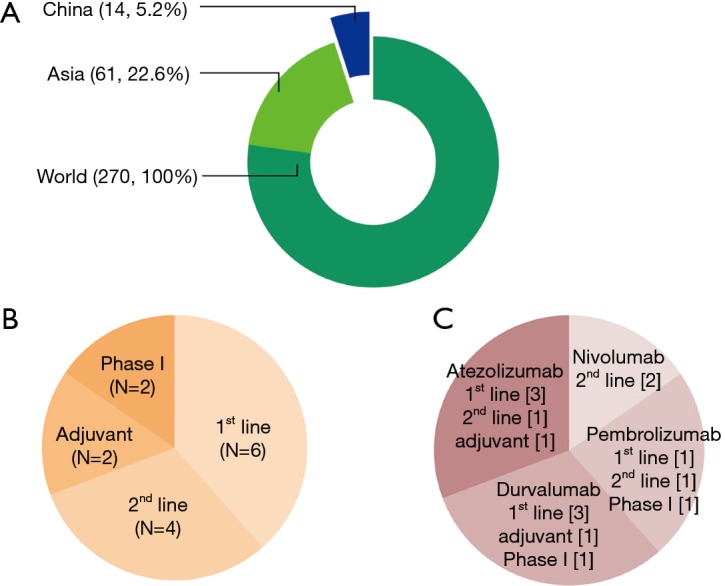
Ongoing international clinical trials including Chinese patients. (A) Between January 01, 2013, and April 06, 2017, there were 270 clinical trials of anti-PD-1/PD-L1 inhibitors for NSCLC that were registered on ClinicalTrials.gov. Among the 270 studies, 61 studies were performed in East Asia and 14 studies were performed in China (12 multinational trials and 2 trials that only evaluated Chinese patients); (B) the 14 clinical trials included six first-line studies, four second-line studies, two adjuvant therapy studies, and two phase I studies for only Chinese patients; (C) the classification of clinical trials in China according to the therapeutic agent, which includes nivolumab, pembrolizumab, atezolizumab, and durvalumab. The image and legend were adopted with permission from reference (8). PD-L1, program death ligand-1; PD-1, program death-1.
