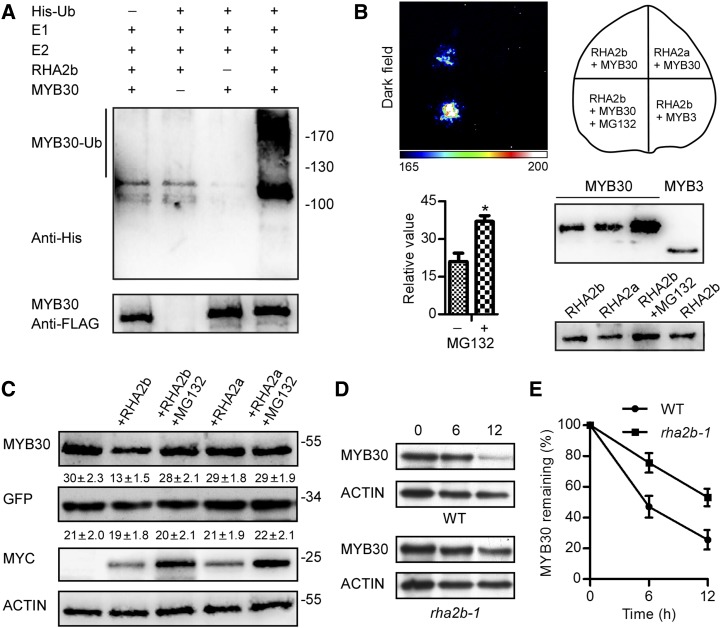
Figure 2.
RHA2b is involved in the degradation of MYB30. A, MYC-RHA2b was capable of ubiquitinating FLAG-MYB30 in vitro in the presence of His-ubiquitin (His-Ub), UBE1, and UbcH5b (E2). MYB30-Ub was analyzed using an anti-His antibody. The loading of MYB30 protein was detected by anti-FLAG antibody. B, Split-luciferase complementation imaging assays in N. benthamiana. The luminescence intensity was analyzed quantitatively. Relative values are presented as means ± sd (n = 3). Higher luminescence intensity was observed after MG132 treatment compared with the control plants (*, P < 0.05, Student’s t test). The expression of protein was detected by anti-LUC antibody. C, RHA2b promoted MYB30 degradation. FLAG-tagged MYB30 and GFP were coexpressed in Arabidopsis protoplasts alone or with MYC-tagged RHA2b or RHA2a and treated with or without 75 μm MG132 as indicated. Densitometry values (means ± se) for FLAG-MYB30 and GFP were calculated from three independent experiments and are shown at the bottom of each immunoblot. ACTIN was used as a loading control of protoplasts. D, ABA-induced MYB30 degradation was delayed in the rha2b-1 mutant. Col-0/35S:FLAG-MYB30 and rha2b-1/35S:FLAG-MYB30 seedlings were pretreated with 100 μm ABA for 24 h and then incubated in the same medium with 100 μm CHX. Samples were collected 0, 6, and 12 h after treatment. WT, Wild-type Col-0. E, MYB30 protein levels were quantified and normalized. Three independent biological repetitions were performed and analyzed. Data presented are means ± sd.