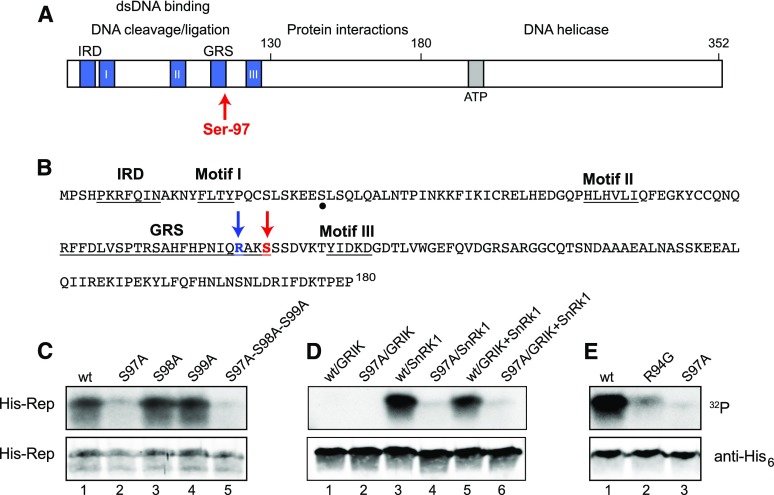
Figure 1.
TGMV Rep is phosphorylated by SnRK1 at Ser-97. A, Schematic diagram of the TGMV Rep showing functional domains, conserved motifs, and Ser-97. B, Amino acid sequence of the TGMV Rep N terminus (1–180). The SnRK1 phosphorylation site Ser-97 and its upstream Arg-94 are indicated by red and blue arrows, respectively. Conserved motifs are underlined. A potential secondary phosphorylation site is marked by a dot. C, Wild-type (wt) His6-Rep(1–131), the S97A, S98A, and S99A single mutants, and the S97A-S98A-S99A triple mutant phosphorylated by GRIK+SnRK1. D, Wild-type His6-Rep(1–180) and its S97A mutant phosphorylated by GRIK, preactivated SnRK1, or GRIK+SnRK1. E, Wild-type His6-Rep(1–180) and the R94G or S97A mutants phosphorylated by preactivated SnRK1. In C to E, recombinant His6-tagged TGMV Rep proteins were incubated in vitro in kinase reactions in the presence of [γ-32P]ATP. The proteins were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. Protein phosphorylation was detected by autoradiography (top gels), and the loading of His6-Rep was assessed by immunoblotting with anti-His6 antibodies (bottom gels).