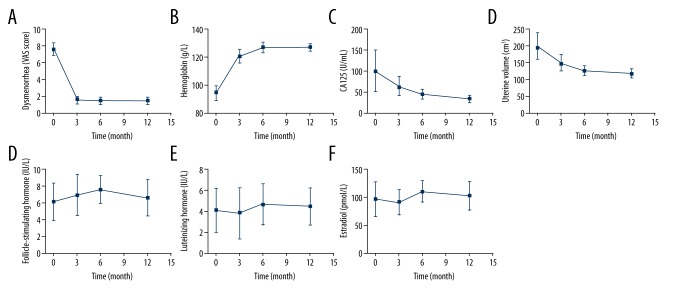
Figure 4.
Changes in dysmenorrhea, hemoglobin, serum cancer antigen 125 (CA125), uterine volume, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels before and after implantation of the subcutaneous device containing 68 mg of etonogestrel. (A) Dysmenorrhea, before and after subcutaneous etonogestrel implantation. (B) Hemoglobin (Hb) levels (g/dL), before and after subcutaneous etonogestrel implantation. (C) Serum cancer antigen 125 (CA125) levels (U/ml), before and after subcutaneous etonogestrel implantation. (D) Uterine volume measurements (mm), before and after subcutaneous etonogestrel implantation. (E) Follicle stimulating hormone (FSH) levels (mIU/mL), before and after subcutaneous etonogestrel implantation. (F) Luteinizing hormone (LH) levels (IU/L), before and after subcutaneous etonogestrel implantation. (G) Estradiol (E2) levels (pg/mL), before and after subcutaneous etonogestrel implantation.