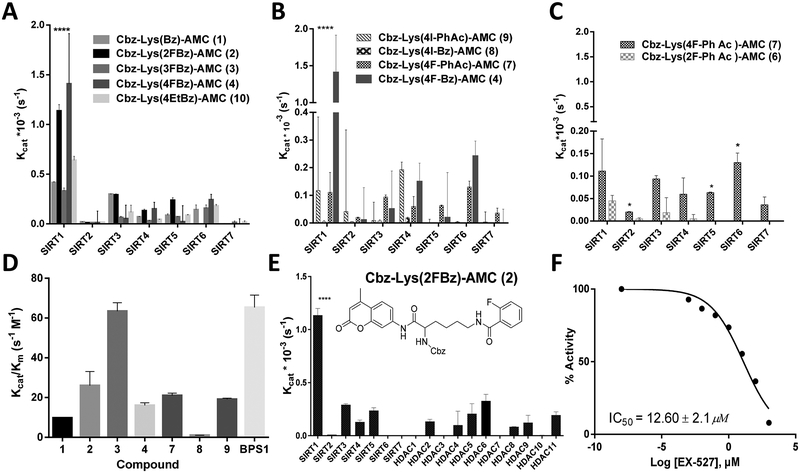
Figure 2.
(A−C) Catalytic efficacy (Kcat, s−1) of compounds 1−10 with SIRT1−7 as determined by an enzymatic assay with a fluorescent readout. (A) Compounds 1−4 and 10 demonstrating differences in Kcat as a result of aromatic substitution of the fluorine atom at varying positions on the benzoyl moiety or the addition of a bulkier ethyl group. (B) Variations in Kcat due to the use of either a benzoyl or phenylacetyl moiety with para positioning of iodine or fluorine, demonstrating a significant increase with para-fluorobenzoyl (4) as compared with those of the others. (C) Comparison of ortho- and para-fluorine substitutions (6 vs 7) on the aromatic ring of a phenylacetyl moiety. (D) Catalytic efficacy (Kcat/Km, s−1 M−1) of selected compounds with recombinant SIRT1, as determined by a fluorometric assay, demonstrating that 2 is cleaved most efficiently by SIRT1. (E) Compound 2 selectively cleaved by SIRT1 with at least 3-fold greater efficacy as compared with by SIRT2−7 and HDAC1−11. (F) SIRT1 cleavage of 2 with increasing levels of EX-527, a selective SIRT1 inhibitor (IC50 = 12.60 ± 2.1 μM). Error bars represent standard deviations. All experiments were performed in triplicate. ****P < 0.01, *P < 0.05.